AZITHROMYCIN
Azithromycin Tablets 600 mg
FULL PRESCRIBING INFORMATION: CONTENTS*
- AZITHROMYCIN DESCRIPTION
- AZITHROMYCIN INDICATIONS AND USAGE
- AZITHROMYCIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- AZITHROMYCIN ADVERSE REACTIONS
- AZITHROMYCIN DOSAGE AND ADMINISTRATION (See AZITHROMYCIN INDICATIONS AND USAGE.)
- HOW SUPPLIED
- CLINICAL STUDIES IN PATIENTS WITH ADVANCED HIV INFECTION FOR THE PREVENTION AND TREATMENT OF DISEASE DUE TO DISSEMINATED COMPLEX (MAC) (See AZITHROMYCIN INDICATIONS AND USAGE)
- ANIMAL TOXICOLOGY
FULL PRESCRIBING INFORMATION
Rx onlyAZITHROMYCIN DESCRIPTION
2R,3S,4R, 5R,8R,10R,11R,12S,13S,14RCOL-riboxylo3872212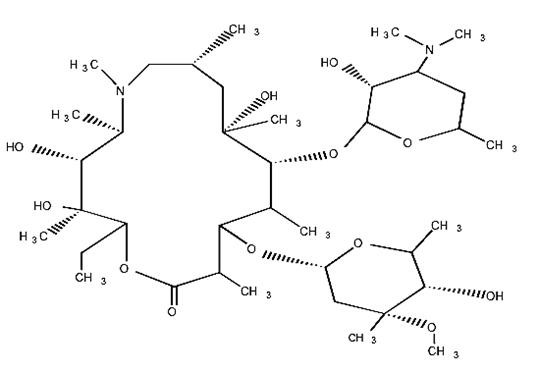 3872212
3872212Pharmacokinetics
| DOSE/DOSAGE FORM (serum, except as indicated) | Subjects | Day No. | Cmax (mcg/mL) | Tmax (hr) | C24 (mcg/mL) | AUC (mcg•hr/mL) | T½ (hr) | Urinary Excretion (% of dose) |
| 500 mg/250 mg capsule | 12 | Day 1 | 0.41 | 2.5 | 0.05 | 2.6a | -- | 4.5 |
| and 250 mg on Days 2-5 | 12 | Day 5 | 0.24 | 3.2 | 0.05 | 2.1a | -- | 6.5 |
| 1200 mg/600 mg tablets | 12 | Day 1 | 0.66 | 2.5 | 0.074 | 6.8b | 40 | -- |
| %CV | (62%) | (79%) | (49%) | (64%) | (33%) | |||
| 600 mg tablet/day | 7 | 1 | 0.33 | 2 | 0.039 | 2.4a | ||
| %CV | (25%) | (50%) | (36%) | (19%) | ||||
| 7 | 22 | 0.55 | 2.1 | 0.14 | 5.8a | 84.5 | -- | |
| %CV | (18%) | (52%) | (26%) | (25%) | -- | |||
| 600 mg tablet/day (leukocytes) | 7 | 22 | 252 | 10.9 | 146 | 4763a | 82.8 | -- |
| %CV | (49%) | (28%) | (33%) | (42%) | -- | -- |
b
minmaxmin
max
max
max
max
| TISSUE OR FLUID | TIME AFTER DOSE (h) | TISSUE OR FLUID CONCENTRATION (mcg/g or mcg/mL)1 | CORRESPONDING PLASMA OR SERUM LEVEL (mcg/mL) | TISSUE (FLUID) PLASMA (SERUM) RATIO |
| SKIN | 72-96 | 0.4 | 0.012 | 35 |
| LUNG | 72-96 | 4 | 0.012 | > 100 |
| SPUTUM* | 2-4 | 1 | 0.64 | 2 |
| SPUTUM** | 10-12 | 2.9 | 0.1 | 30 |
| TONSIL*** | 9-18 | 4.5 | 0.03 | > 100 |
| TONSIL*** | 180 | 0.9 | 0.006 | > 100 |
| CERVIX**** | 19 | 2.8 | 0.04 | 70 |
*
**
***
****
max
max
Renal Insufficiency
max0-120max0-120DOSAGE AND ADMINISTRATIONHepatic Insufficiency
Mechanism of Action
in vitro In vivo
Microbiology
in vitroAerobic Gram-Positive Microorganisms
Staphylococcus aureus
Streptococcus agalactiae
Streptococcus pneumoniae
Streptococcus pyogenes
Enterococcus faecalis
Aerobic Gram-Negative Microorganisms
Haemophilus influenzae
Moraxella catarrhalis
“Other” Microorganisms
Chlamydia trachomatis
in vitro
Mycobacteria
Mycobacterium avium
Mycobacterium avium
Mycobacterium intracellulare.
in vitrobut their clinical significance is unknown.
in vitro
Aerobic Gram-Positive Microorganisms
Aerobic Gram-Negative Microorganisms
Bordetella pertussis
Campylobacter jejuni
Haemophilus ducreyi
Legionella pneumophila
Anaerobic Microorganisms
Bacteroides bivius
Clostridium perfringens
Peptostreptococcus species
“Other” Microorganisms
Borrelia burgdorferi
Mycoplasma pneumoniae
Treponema pallidum
Ureaplasma urealyticum
Susceptibility Testing of Bacteria Excluding Mycobacteria
in vitro2in vitro2in vitro
Dilution Techniques :
1
| MIC (mcg/mL) | Interpretation |
| ≤ 2 | Susceptible (S) |
| 4 | Intermediate (I) |
| ≥ 8 | Resistant (R) |
| Microorganism | MIC (mcg/mL) |
| Escherichia coli ATCC 25922 | 2.0-8.0 |
| Enterococcus faecalis ATCC 29212 | 1.0-4.0 |
| Staphylococcus aureus ATCC 29213 | 0.25-1.0 |
2
| Zone Diameter (mm) | Interpretation |
| ≥ 18 | (S) Susceptible |
| 14-17 | (I) Intermediate |
| ≤ 13 | (R) Resistant |
| Microorganism | Zone Diameter (mm) |
| Staphylococcus aureus ATCC 25923 | 21-26 |
in vitroMycobacterium aviumM. aviumM. intracellulare,M. aviumM. avium
in vitroMycobacterium avium
Drug Resistance
Mycobacterium aviumEscherichia coli 3,4Mycobacterium aviumM. aviumSusceptibility testing for Mycobacterium avium complex (MAC):
In vitroMycobacterium aviumM. aviumM. intracellulare
in vitroMycobacteriumtuberculosis
AZITHROMYCIN INDICATIONS AND USAGE
Mycobacterial Infections
Prophylaxis of Disseminated M ycobacterium avium complex (MAC) Disease
Mycobacterium avium
Treatment of Disseminated Mycobacterium avium complex (MAC) Disease
AZITHROMYCIN CONTRAINDICATIONS
WARNINGS
CONTRAINDICATIONSrecurred soon thereafter in some patients without further azithromycin exposureClostridium difficileC. difficile.
C. difficileC. difficile
C. difficileC. difficile
PRECAUTIONS
General
CLINICAL PHARMACOLOGY - Renal Insufficiencytorsades de pointes
Information for Patients
Drug Interactions
max
0-8max
0-∞max
thmax
max
max
maxmaxmaxmax
max
450
Laboratory Test Interactions:
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Pregnancy: 2
2
2
Nursing Mothers:
Pediatric Use:
Safety in HIV-Infected Pediatric Patients:
Geriatric Use:
Geriatric Patients with Opportunistic Infections, Including Mycobacterium avium complex (MAC) Disease:
AZITHROMYCIN ADVERSE REACTIONS
Clinical
Multiple-dose regimen:Cardiovascular:
Gastrointestinal:
Genitourinary:
Nervous System:
General:
Allergic:
Chronic therapy with 1200 mg weekly regimen:Mycobacterium avium
Chronic therapy with 600 mg daily regimen combined with ethambutol:
Single 1-gram dose regimen:
Post-Marketing Experience
Allergic:
Cardiovascular: torsades de pointes.
Gastrointestinal:
General:
Genitourinary:
Hematopoietic:
Liver/Biliary:
Nervous System:
Psychiatric:
Skin/Appendages:
Special Senses:
Laboratory Abnormalities
DOSAGE AND ADMINISTRATION (See INDICATIONS AND USAGE.)
Pediatric Use
Prevention of Disseminated MAC Infections
Mycobacterium avium
Treatment of Disseminated MAC Infections
in vitro
0-120CLINICAL PHARMACOLOGY-Renal Insufficiency.
CLINICAL PHARMACOLOGY-Hepatic Impairment.
HOW SUPPLIED
CLINICAL STUDIES IN PATIENTS WITH ADVANCED HIV INFECTION FOR THE PREVENTION AND TREATMENT OF DISEASE DUE TO DISSEMINATED COMPLEX (MAC) (See INDICATIONS AND USAGE)
Prevention of Disseminated MAC Disease
MAC bacteremia
| Cumulative Incidence Rate, %: Placebo (n = 89) | ||||
| Month | MAC Free and Alive | MAC | Adverse Experience | Lost to Follow-up |
| 6 | 69.7 | 13.5 | 6.7 | 10.1 |
| 12 | 47.2 | 19.1 | 15.7 | 18 |
| 18 | 37.1 | 22.5 | 18 | 22.5 |
| Cumulative Incidence Rate, %: Azithromycin (n = 85) | ||||
| Month | MAC Free and Alive | MAC | Adverse Experience | Lost to Follow-up |
| 6 | 84.7 | 3.5 | 9.4 | 2.4 |
| 12 | 63.5 | 8.2 | 16.5 | 11.8 |
| 18 | 44.7 | 11.8 | 25.9 | 17.6 |
| Cumulative Incidence Rate, %: Rifabutin (n = 223) | ||||
| Month | MAC Free and Alive | MAC | Adverse Experience | Lost to Follow-up |
| 6 | 83.4 | 7.2 | 8.1 | 1.3 |
| 12 | 60.1 | 15.2 | 16.1 | 8.5 |
| 18 | 40.8 | 21.5 | 24.2 | 13.5 |
| Cumulative Incidence Rate, %: Azithromycin (n = 223) | ||||
| Month | MAC Free and Alive | MAC | Adverse Experience | Lost to Follow-up |
| 6 | 85.2 | 3.6 | 5.8 | 5.4 |
| 12 | 65.5 | 7.6 | 16.1 | 10.8 |
| 18 | 45.3 | 12.1 | 23.8 | 18.8 |
| Cumulative Incidence Rate, %: Azithromycin/Rifabutin Combination (n = 218) | ||||
| Month | MAC Free and Alive | MAC | Adverse Experience | Lost to Follow-up |
| 6 | 89.4 | 1.8 | 5.5 | 3.2 |
| 12 | 71.6 | 2.8 | 15.1 | 10.6 |
| 18 | 49.1 | 6.4 | 29.4 | 15.1 |
5
Clinically Significant Disseminated MAC Disease
Discontinuations From Therapy For Drug-Related Side Effects
Safety
| Study 155 | Study 174 | ||||
| Placebo | Azithromycin 1200 mg weekly | Azithromycin 1200 mg weekly | Rifabutin 300 mg daily | Azithromycin + Rifabutin | |
| (N = 91) | (N = 89) | (N = 233) | (N = 236) | (N = 224) | |
| Mean Duration of Therapy (days) | 303.8 | 402.9 | 315 | 296.1 | 344.4 |
| Discontinuation of Therapy | 2.3 | 8.2 | 13.5 | 15.9 | 22.7 |
| Autonomic Nervous System | |||||
| Mouth Dry | 0 | 0 | 0 | 3 | 2.7 |
| Central Nervous System | |||||
| Dizziness | 0 | 1.1 | 3.9 | 1.7 | 0.4 |
| Headache | 0 | 0 | 3 | 5.5 | 4.5 |
| Gastrointestinal | |||||
| Diarrhea | 15.4 | 52.8 | 50.2 | 19.1 | 50.9 |
| Loose Stools | 6.6 | 19.1 | 12.9 | 3 | 9.4 |
| Abdominal Pain | 6.6 | 27 | 32.2 | 12.3 | 31.7 |
| Dyspepsia | 1.1 | 9 | 4.7 | 1.7 | 1.8 |
| Flatulence | 4.4 | 9 | 10.7 | 5.1 | 5.8 |
| Nausea | 11 | 32.6 | 27 | 16.5 | 28.1 |
| Vomiting | 1.1 | 6.7 | 9 | 3.8 | 5.8 |
| General | |||||
| Fever | 1.1 | 0 | 2.1 | 4.2 | 4.9 |
| Fatigue | 0 | 2.2 | 3.9 | 2.1 | 3.1 |
| Malaise | 0 | 1.1 | 0.4 | 0 | 2.2 |
| Musculoskeletal | |||||
| Arthralgia | 0 | 0 | 3 | 4.2 | 7.1 |
| Psychiatric | |||||
| Anorexia | 1.1 | 0 | 2.1 | 2.1 | 3.1 |
| Skin & Appendages | |||||
| Pruritus | 3.3 | 0 | 3.9 | 3.4 | 7.6 |
| Rash | 3.2 | 3.4 | 8.1 | 9.4 | 11.1 |
| Skin discoloration | 0 | 0 | 0 | 2.1 | 2.2 |
| Special Senses | |||||
| Tinnitus | 4.4 | 3.4 | 0.9 | 1.3 | 0.9 |
| Hearing Decreased | 2.2 | 1.1 | 0.9 | 0.4 | 0 |
| Uveitis | 0 | 0 | 0.4 | 1.3 | 1.8 |
| Taste Perversion | 0 | 0 | 1.3 | 2.5 | 1.3 |
Changes in Laboratory Values
| Placebo | Azithromycin 1200 mg weekly | Rifabutin 300 mg daily | Azithromycin & Rifabutin | ||||||
| Hemoglobin | < 8 g/dl | 1/51 | 2% | 4/170 | 2% | 4/114 | 4% | 8/107 | 8% |
| Platelet Count | < 50 x 103 /mm3 | 1/71 | 1% | 4/260 | 2% | 2/182 | 1% | 6/181 | 3% |
| WBC Count | < 1 x 103 /mm3 | 0/8 | 0% | 2/70 | 3% | 2/47 | 4% | 0/43 | 0% |
| Neutrophils | < 500/mm3 | 0/26 | 0% | 4/106 | 4% | 3/82 | 4% | 2/78 | 3% |
| SGOT | > 5 x ULNa | 1/41 | 2% | 8/158 | 5% | 3/121 | 3% | 6/114 | 5% |
| SGPT | > 5 x ULN | 0/49 | 0% | 8/166 | 5% | 3/130 | 2% | 5/117 | 4% |
| Alk Phos | > 5 x ULN | 1/80 | 1% | 4/247 | 2% | 2/172 | 1% | 3/164 | 2% |
Treatment of Disseminated MAC Disease
| Azithromycin 600 mg qd | Clarithromycin 500 mg bid | **95.1% CI on difference | |
| Patients with positive culture at baseline | 68 | 57 | |
| Week 24 | |||
| Two consecutive negative blood cultures* | 31/68 (46%) | 32/57 (56%) | [-28, 7] |
| Mortality | 16/68 (24%) | 15/57 (26%) | [-18, 13] |
Sterilization by Baseline Colony Count
| Azithromycin 600 mg (N = 68) | Clarithromycin 500 mg bid (N = 57) | |
| Groups Stratified by MAC Colony Counts at Baseline | No. (%) Subjects in Stratified Group Sterile at Week 24 | No. (%) Subjects in Stratified Group Sterile at Week 24 |
| ≤ 10 cfu/mL | 10/15 (66.7%) | 12/17 (70.6%) |
| 11-100 cfu/mL | 13/28 (46.4%) | 13/19 (68.4%) |
| 101-1,000 cfu/mL | 7/19 (36.8%) | 5/13 (38.5%) |
| 1,001-10,000 cfu/mL | 1/5 (20.0%) | 1/5 (20%) |
| > 10,000 cfu/mL | 0/1 (0.0%) | 1/3 (33.3%) |
Susceptibility Pattern of MAC Isolates
ANIMAL TOXICOLOGY
REFERENCES:
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically-Third Edition. Approved Standard NCCLS Document M7-A3, Vol. 13, No. 25, NCCLS, Villanova, PA, December 1993.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests-Fifth Edition. Approved Standard NCCLS Document M2-A5, Vol. 13, No. 24, NCCLS, Villanova, PA, December 1993.
- Dunne MW, Foulds G, Retsema JA. Rationale for the use of azithromycin as Mycobacterium avium chemoprophylaxis. American J Medicine 1997; 102(5C):37-49.
- Meier A, Kirshner P, Springer B, et al. Identification of mutations in 23S rRNA gene of clarithromycin-resistant Mycobacterium intracellulare. Antimicrob Agents Chemother. 1994;38:381-384.
- Methodology per Inderlied CB, et al. Determination of In Vitro Susceptibility of Mycobacterium avium Complex Isolates to Antimicrobial Agents by Various Methods. Antimicrob Agents Chemother 1987; 31:1697-1702.
Manufactured by:
Distributed by:

AZITHROMYCINAZITHROMYCIN TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!