Azilect
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AZILECT® safely and effectively. See full prescribing information for AZILECT®. AZILECT® (rasagiline mesylate) Tablets for Oral Use Initial U.S. Approval: 2006 RECENT MAJOR CHANGES Dosage and Administration 12/2009 Contraindications 12/2009 Warnings and Precautions 12/2009 INDICATIONS AND USAGEAZILECT is indicated for the treatment of the signs and symptoms of idiopathic Parkinson's disease as initial monotherapy and as adjunct therapy to levodopa. (1) DOSAGE AND ADMINISTRATION Monotherapy: AZILECT 1 mg once daily (2.1) As adjunct to levodopa: AZILECT 0.5 mg once daily. Dose increase to 1 mg daily as required for sufficient clinical response. (2.2) Patients with mild hepatic impairment: AZILECT 0.5 mg once daily should not be exceeded. AZILECT should not be used in patients with moderate or severe hepatic impairment (2.3) AZILECT has not been studied in patients with severe renal impairment (2.4) Patients taking ciprofloxacin or other CYP1A2 inhibitors: AZILECT 0.5 mg once daily should not be exceeded. (2.5) DOSAGE FORMS AND STRENGTHS AZILECT 0.5 mg tablets (containing, as the active ingredient, rasagiline mesylate equivalent to 0.5 mg of rasagiline base) (3) AZILECT 1 mg tablets (containing, as the active ingredient, rasagiline mesylate equivalent to 1 mg of rasagiline base) (3) CONTRAINDICATIONS Concomitant use of : -meperidine, tramadol, methadone or propoxyphene (4.1)-dextromethorphan, St. John's wort or cyclobenzaprine (4.2)-other MAO inhibitors (selective or non-selective) (4.3) WARNINGS AND PRECAUTIONS Risk of severe CNS toxicity (serotonin syndrome) when AZILECT is combined with antidepressants. (5.1) Concomitant use of ciprofloxacin or other CYP1A2 inhibitors: Increase in rasagiline plasma concentrations. 0.5 mg rasagiline once daily should not be exceeded (5.2) Patients with hepatic impairment: Increase in rasagiline plasma concentrations. Limit dose to 0.5 mg rasagiline in mild hepatic impairment. AZILECT should not be used in patients with moderate or severe hepatic impairment (5.3) Risk for Hypertensive Crisis and nonselective MAO inhibition above the recommended Doses (5.4) Melanoma (5.4) AZILECT may cause lower blood pressure, especially postural hypotension (5.7) or increase blood pressure in different patients (5.8) AZILECT may cause or exacerbate hallucinations or potentially other manifestations of psychotic-like behavior (5.9) Side Effects Most common adverse reactions (treatment difference ≥ 3% greater than placebo); with monotherapy: flu syndrome, arthralgia, depression, dyspepsia. (6.1) Most common adverse reactions (treatment difference ≥ 3% greater than placebo); when used as adjunct to levodopa: dyskinesia, accidental injury, weight loss, postural hypotension, vomiting, anorexia, arthralgia, abdominal pain, nausea, constipation, dry mouth, rash, abnormal dreams, fall. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact TEVA at 1-800-221-4026 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Meperidine: Risk of serious, sometimes fatal reactions from serotonin syndrome. See also Contraindications. (7.1) Dextromethorphan: Risk of psychosis episodes or bizarre behavior. See also Contraindications. (7.2) MAO inhibitors: Risk of non-selective MAO inhibition and hypertensive crisis. See also Contraindications. (7.4) Antidepressants (SSRIs, SNRIs, tricyclic, tetracyclic, or triazolopyridine): Concomitant use not recommended. (7.5) Levodopa: See also Warnings and Precautions. (7.6) Ciprofloxacin and Other CYP1A2 Inhibitors: Increased rasagiline plasma levels possible. Increased risk of adverse events. See also Dosage and Administration and Warnings and Precautions.(7.7) USE IN SPECIFIC POPULATIONS Pregnancy: AZILECT should be used only if the potential benefit justifies the potential risk to the fetus. (8.1) Nursing mothers: Rasagiline inhibits prolactin secretion and may inhibit milk secretion. It is not known whether rasagiline is excreted in human milk. Use with caution. (8.3) Hepatic impairment: Rasagiline plasma concentrations may be increased. See also Dosage and Administration and Warnings and Precautions. (8.6)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 AZILECT INDICATIONS AND USAGE
- 2 AZILECT DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 AZILECT CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 5.1 Coadministration with Antidepressants
- 5.2 Ciprofloxacin and Other CYP1A2 Inhibitors
- 5.3 Hepatic Impairment
- 5.4 Risk for Hypertensive Crisis and Nonselective Monoamine Oxidase Inhibition Above The Recommended Doses
- 5.5 Melanoma
- 5.6 Dyskinesia
- 5.7 Lowering of Blood Pressure and Postural/Orthostatic Hypotension
- 5.8 Elevation of Blood Pressure
- 5.9 Hallucinations / Psychotic-Like Behavior
- 5.10 Withdrawal-Emergent Hyperpyrexia and Confusion
- 5.11 Laboratory Tests
- 6 AZILECT ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 AZILECT DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL TRIALS
- 16 HOW SUPPLIED
- 17 INFORMATION FOR PATIENTS
- 17.1 Coadministration of Antidepressants and Other Drugs
- 17.2 Ciprofloxacin or Other CYP1A2 Inhibitors
- 17.3 Risk of Hypertensive Crisis and Nonselective Monoamine Oxidase Inhibition Above the Recommended Doses
- 17.4 Melanoma
- 17.5 Dyskinesia
- 17.6 Lowering of Blood Pressure and Postural/Orthostatic Hypotension
- 17.7 Elevation of Blood Pressure
- 17.8 Hallucinations / Psychotic-Like Behavior
- 17.9 Withdrawal-Emergent Hyperpyrexia and Confusion
- 17.10 Missing Dose
- 17.11 Impulse Control / Compulsive Behaviors
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Enter section text here
1 INDICATIONS AND USAGE
AZILECT (rasagiline tablets) is indicated for the treatment of the signs and symptoms of idiopathic Parkinson's disease as initial monotherapy and as adjunct therapy to levodopa.
The effectiveness of AZILECT was demonstrated in patients with early Parkinson's disease who were receiving AZILECT as monotherapy and who were not receiving any concomitant dopaminergic therapy. The effectiveness of AZILECT as adjunct therapy was demonstrated in patients with Parkinson's disease who were treated with levodopa.
2 DOSAGE AND ADMINISTRATION
AZILECT is a selective inhibitor of monoamine oxidase (MAO)-B at recommended doses of 0.5 or 1 mg daily. Dietary tyramine restriction is not ordinarily required with recommended doses of AZILECT. However, certain foods (e.g., aged cheeses, such as Stilton cheese) may contain very high amounts (i.e., > 150 mg) of tyramine and could potentially cause a hypertensive "cheese" reaction in patients taking AZILECT even at the recommended dose due to mild increased sensitivity to tyramine. The selectivity for inhibiting MAO-B diminishes in a dose-related manner as the dose is progressively increased above the recommended daily dose [see Warnings and Precautions (5.4), Clinical Pharmacology (12.3, and Information for Patients (17.3))].
2.1 Monotherapy
The recommended AZILECT dose for the treatment of Parkinson's disease patients is 1 mg administered orally once daily.
2.2 Adjunctive Therapy
The recommended initial dose is 0.5 mg administered orally once daily. If a sufficient clinical response is not achieved, the dose may be increased to 1 mg administered once daily.
Change of Levodopa Dose in Adjunct Therapy
When AZILECT is used in combination with levodopa, a reduction of the levodopa dosage may be considered based upon individual response. During the controlled trials of AZILECT as adjunct therapy to levodopa, levodopa dosage was reduced in some patients. In clinical studies, dosage reduction of levodopa was allowed within the first 6 weeks if dopaminergic side effects, including dyskinesia and hallucinations, emerged. In Study 1, levodopa dosage reduction occurred in 8% of patients in the placebo group and in 16% and 17% of patients in the 0.5 mg/day and 1 mg/day rasagiline groups, respectively. In those patients who had levodopa dosage reduced, the dose was reduced on average by about 7%, 9%, and 13% in the placebo, 0.5 mg/day, and 1 mg/day groups, respectively. In Study 2, levodopa dosage reduction occurred in 6% of patients in the placebo group and in 9% in the rasagiline 1 mg/day group. In patients who had their levodopa dosage reduced, the dose was reduced on average by about 13% and 11% in the placebo and the rasagiline groups, respectively.
2.3 Patients with Hepatic Impairment
AZILECT plasma concentrations will increase in patients with hepatic impairment. Patients with mild hepatic impairment should use 0.5 mg daily of AZILECT. AZILECT should not be used in patients with moderate or severe hepatic impairment [see Warnings and Precautions (5.3), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
2.4 Patients with Renal Impairment
Dose adjustment of AZILECT is not required for patients with mild or moderate renal impairment because AZILECT plasma concentrations are not increased in patients with moderate renal impairment. Rasagiline has not been studied in patients with severe renal impairment.
2.5 Patients Taking Ciprofloxacin or Other CYP1A2 Inhibitors
Rasagiline plasma concentrations are expected to double in patients taking concomitant ciprofloxacin and other CYP1A2 inhibitors. Therefore, patients taking concomitant ciprofloxacin or other CYP1A2 inhibitors should use 0.5 mg daily of AZILECT [see Warnings and Precautions (5.2), Drug Interactions (7.7), and Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
AZILECT 0.5 mg Tablets: White to off-white, round, flat, beveled tablets, debossed with "GIL 0.5" on one side and plain on the other side containing, as the active ingredient, rasagiline mesylate equivalent to 0.5 mg of rasagiline base.
AZILECT 1 mg Tablets: White to off-white, round, flat, beveled tablets, debossed with "GIL 1" on one side and plain on the other side containing, as the active ingredient, rasagiline mesylate equivalent to 1 mg of rasagiline base.
4 CONTRAINDICATIONS
Enter section text here
4.1 Meperidine and Certain Other Analgesics
AZILECT is contraindicated for use with meperidine. Serious adverse reactions have been precipitated with concomitant use of meperidine (e.g., Demerol and other tradenames) and MAO inhibitors (MAOIs) including selective MAO-B inhibitors. These adverse reactions are often described as "serotonin syndrome", a potentially serious condition, which can result in death. Typical clinical signs and symptoms include behavioral and cognitive/mental status changes (e.g., confusion, hypomania, hallucinations, agitation, delirium, headache, and coma), autonomic effects (e.g., syncope, shivering, sweating, high fever/hyperthermia, hypertension, hypotension, tachycardia, nausea, diarrhea), and somatic effects (e.g., muscular rigidity, myoclonus, muscle twitching, hyperreflexia manifested by clonus, and tremor). At least 14 days should elapse between discontinuation of AZILECT and initiation of treatment with meperidine.
For similar reasons, AZILECT should not be administered with the analgesic agents tramadol, methadone, and propoxyphene.
In the post-marketing period, serotonin syndrome has been reported in a patient erroneously treated with a higher than recommended dose of AZILECT (4 mg daily) and tramadol.
4.2 Other Drugs
AZILECT should not be used with the antitussive agent dextromethorphan. The combination of MAO inhibitors and dextromethorphan has been reported to cause brief episodes of psychosis or bizarre behavior. AZILECT is also contraindicated for use with St. John's wort, and cyclobenzaprine (a tricyclic muscle relaxant).
4.3 MAO Inhibitors
AZILECT should not be administered along with any other MAO inhibitor (selective or non-selective) because of the increased risk of non-selective MAO inhibition that may lead to a hypertensive crisis. At least 14 days should elapse between discontinuation of AZILECT and initiation of treatment with any MAO inhibitor.
5 WARNINGS AND PRECAUTIONS
Enter section text here
5.1 Coadministration with Antidepressants
Severe CNS toxicity associated with hyperpyrexia has been reported with the combined treatment of an antidepressant (e.g., selective serotonin reuptake inhibitors-SSRIs, serotonin-norepinephrine reuptake inhibitors-SNRIs, tricyclic antidepressants, tetracyclic antidepressants, triazolopyridine antidepressants) and a non-selective MAOI (e.g., phenelzine, tranylcypromine) or selective MAO-B inhibitors, such as selegiline (Eldepryl) and rasagiline (AZILECT). These adverse reactions are often described as "serotonin syndrome" which can result in death. In the post-marketing period, non-fatal cases of serotonin syndrome have been reported in patients treated with antidepressants concomitantly with AZILECT.
The symptoms of serotonin syndrome have included behavioral and cognitive/mental status changes (e.g., confusion, hypomania, hallucinations, agitation, delirium, headache, and coma), autonomic effects (e.g., syncope, shivering, sweating, high fever/hyperthermia, hypertension, tachycardia, nausea, diarrhea), and somatic effects (e.g., muscular rigidity, myoclonus, muscle twitching, hyperreflexia manifested by clonus, and tremor).
AZILECT clinical trials did not allow concomitant use of fluoxetine or fluvoxamine with AZILECT, but the following antidepressants and doses were allowed in the AZILECT trials: amitriptyline ≤ 50 mg/daily, trazodone ≤ 100 mg/daily, citalopram ≤ 20 mg/daily, sertraline ≤ 100 mg/daily and paroxetine ≤ 30 mg/daily.
Although a small number of rasagiline-treated patients were concomitantly exposed to antidepressants (tricyclics n=115; SSRIs n=141), the exposure, both in dose and number of subjects, was not adequate to rule out the possibility of an untoward reaction from combining these agents. Furthermore, because the mechanisms of these reactions are not fully understood, it seems prudent, in general, to avoid the combination of AZILECT with any antidepressant. At least 14 days should elapse between discontinuation of AZILECT and initiation of treatment with a SSRI, SNRI, tricyclic, tetracyclic, or triazolopyridine antidepressant. Because of the long half lives of certain antidepressants (e.g., fluoxetine and its active metabolite), at least five weeks (perhaps longer, especially if fluoxetine has been prescribed chronically and/or at higher doses) should elapse between discontinuation of fluoxetine and initiation of AZILECT [see Drug Interactions (7.5)].
5.2 Ciprofloxacin and Other CYP1A2 Inhibitors
Rasagiline plasma concentrations may increase up to 2 fold in patients using concomitant ciprofloxacin and other CYP1A2 inhibitors [see Dosage and Administration (2.5), Drug Interactions (7.7), and Clinical Pharmacology (12.3)].
5.3 Hepatic Impairment
Rasagiline plasma concentration may increase in patients with mild (up to 2 fold, Child-Pugh score 5-6), moderate (up to 7 fold, Child-Pugh score 7-9), and severe (Child-Pugh score 10-15) hepatic impairment. Patients with mild hepatic impairment should be given the dose of 0.5 mg/day. AZILECT should not be used in patients with moderate or severe hepatic impairment [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
5.4 Risk for Hypertensive Crisis and Nonselective Monoamine Oxidase Inhibition Above The Recommended Doses
AZILECT is a selective inhibitor of monoamine oxidase (MAO)-B at the recommended doses of 0.5 or 1 mg daily. AZILECT should not be used at daily doses exceeding 1 mg/day (or 0.5 mg/day for patients with mild hepatic impairment or in patients using concomitant ciprofloxacin or another CYP1A2 inhibitor) because of the risks of hypertensive crisis and other adverse reactions associated with nonselective inhibition of MAO [see Dosage and Administration (2), Drug Interactions (7.9), and Clinical Pharmacology (12.3)].
Dietary tyramine restriction is not ordinarily required with ingestion of most foods and beverages that may contain tyramine, during treatment with recommended doses of AZILECT. However, certain foods (e.g., aged cheeses, such as Stilton cheese) may contain very high amounts (i.e., > 150 mg) of tyramine and could potentially cause a hypertensive "cheese" reaction in patients taking AZILECT even at the recommended doses due to mild increased sensitivity to tyramine. Patients should be advised to avoid foods (e.g., aged cheese) containing a very large amount of tyramine while taking recommended doses of AZILECT because of the potential for large increases in blood pressure. Selectivity for inhibiting MAO-B diminishes in a dose-related manner as the dose is progressively increased above the recommended daily doses.
There were no cases of hypertensive crisis in the clinical development program associated with 1 mg daily rasagiline treatment, in which most patients did not follow dietary tyramine restriction.
Rare cases of hypertensive crisis have been reported in the post-marketing period in patients after ingesting unknown amounts of tyramine-rich foods while taking recommended doses of AZILECT.
5.5 Melanoma
Epidemiological studies have shown that patients with Parkinson's disease have a higher risk (2- to approximately 6-fold higher) of developing melanoma than the general population. Whether the increased risk observed was due to Parkinson's disease or other factors, such as drugs used to treat Parkinson's disease, is unclear.
For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g., dermatologists).
5.6 Dyskinesia
When used as an adjunct to levodopa, AZILECT may cause dyskinesia or potentiate dopaminergic side effects and exacerbate pre-existing dyskinesia (treatment-emergent dyskinesia occurred in about 18% of patients treated with 0.5 mg or 1 mg rasagiline as an adjunct to levodopa, and 10% of patients who received placebo as an adjunct to levodopa). Decreasing the dose of levodopa may ameliorate this side effect.
5.7 Lowering of Blood Pressure and Postural/Orthostatic Hypotension
In placebo controlled studies of AZILECT given in combination with levodopa, the incidence of postural hypotension consisting of a systolic blood pressure decrease (> 30 mm Hg) or a diastolic blood pressure decrease (> 20 mm Hg) after standing was 13.4 % with AZILECT (1 mg/day) compared to 8.5 % with placebo.
At the 1 mg dose, the frequency of orthostatic hypotension at any time during the study was approximately 44 % for AZILECT vs 33% for placebo for mild to moderate systolic blood pressure decrements (> 20 mm Hg), 40 % for AZILECT vs 33 % for placebo for mild to moderate diastolic blood pressure decrements (> 10 mm Hg), 7 % for AZILECT vs 3 % for placebo for severe systolic blood pressure decrements (> 40 mm Hg), and 9 % for AZILECT vs 6 % for placebo for severe diastolic blood pressure decrements (> 20 mm Hg). There was also an increased risk for some of these abnormalities at the lower 0.5 mg daily dose and for an individual patient having mild to moderate or severe postural hypotension for both systolic and diastolic blood pressure.
Clinical trial data further suggest that postural hypotension occurs most frequently in the first two months of AZILECT treatment and tends to decrease over time.
Some patients treated with AZILECT experienced a mildly increased risk for significant decreases in blood pressure unrelated to standing but while supine.
The risk for post-treatment hypotension (e.g., systolic < 90 or diastolic < 50 mm Hg) combined with a significant decrease from baseline (e.g., systolic > 30 or diastolic > 20 mm Hg) was higher for AZILECT 1 mg (3.2 %) compared to placebo (1.3 %).
There was no clear increased risk for lowering of blood pressure or postural hypotension associated with AZILECT 1 mg/day as monotherapy.
When used as an adjunct to levodopa, postural hypotension was also reported as an adverse reaction in approximately 6% of patients treated with 0.5 mg rasagiline, 9% of patients treated with 1 mg rasagiline and 3% of patients treated with placebo. Postural hypotension led to drug discontinuation and premature withdrawal from clinical trials in one (0.7%) patient treated with rasagiline 1 mg/day, no patients treated with rasagiline 0.5 mg/day and no placebo-treated patients.
5.8 Elevation of Blood Pressure
In studies in which AZILECT (1 mg/day) was given in conjunction with levodopa, AZILECT produced an increased incidence of a significant, high blood pressure (e.g., systolic > 180 or diastolic > 100 mm Hg) of 4% compared to 3% for placebo.
The risk for developing post-treatment high blood pressure (e.g., systolic > 180 or diastolic >100 mm Hg) combined with a significant increase from baseline (e.g., systolic > 30 or diastolic > 20 mm Hg) was higher for AZILECT (2 %) compared to placebo (1 %).
There was no increased frequency of the incidence of hypertension as an adverse reaction in the adjunctive treatment pivotal trials for AZILECT treatment vs placebo.
There was no observed increased risk for increasing blood pressure or high blood pressure (based upon various measurements and analyses) or for the development of hypertension as an adverse reaction in the monotherapy study for 1 mg daily AZILECT treatment (vs placebo).
5.9 Hallucinations / Psychotic-Like Behavior
In the monotherapy study, hallucinations were reported as an adverse event in 1.3% of patients treated with 1 mg rasagiline and in 0.7% of patients treated with placebo. In the monotherapy trial, hallucinations led to drug discontinuation and premature withdrawal from clinical trials in 1.3% of the 1 mg rasagiline-treated patients and in none of the placebo-treated patients.
When used as an adjunct to levodopa, hallucinations were reported as an adverse reaction in approximately 5% of patients treated with 0.5 mg/day AZILECT, 4% of patients treated with 1 mg/day AZILECT and 3% of patients treated with placebo. Hallucinations led to drug discontinuation and premature withdrawal from clinical trials in about 1% of patients treated with 0.5 mg/day or 1 mg/day rasagiline and none of the placebo-treated patients.
Patients should be informed of the possibility of developing hallucinations and instructed to report them to their health care provider promptly should they develop.
Patients with a major psychotic disorder should ordinarily not be treated with AZILECT because of the risk of exacerbating the psychosis with an increase in central dopaminergic tone. In addition, many treatments for psychosis that decrease in central dopaminergic tone may decrease the effectiveness of AZILECT.
AZILECT administration may cause or exacerbate psychotic-like behavior based upon post-marketing reports. This adverse reaction has been reported with many anti-Parkinsonian drugs that increase central dopaminergic tone. This abnormal behavior has been exhibited by one or more of a variety of manifestations including paranoia, confusional state/confusion, psychotic disorder, agitation, delusion, and hallucinations.
5.10 Withdrawal-Emergent Hyperpyrexia and Confusion
A symptom complex resembling neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in drugs that increase central dopaminergic tone. [see Dosage and Administration (2.2)].
Withdrawal emergent hyperpyrexia was not reported in the AZILECT clinical development program.
5.11 Laboratory Tests
No specific laboratory tests are required for the treatment of patients on AZILECT.
6 ADVERSE REACTIONS
Enter section text here
6.1 Clinical Studies Experience
During the clinical development of AZILECT, 1361 Parkinson's disease patients received rasagiline as initial monotherapy or as adjunct therapy to levodopa. As these two populations differ, not only in the adjunct use of levodopa during rasagiline treatment, but also in the severity and duration of their disease, they may have differential risks for various adverse reactions. Therefore, most of the adverse reactions data in this section are presented separately for each population.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates of adverse reactions observed in practice.
Patients Receiving AZILECT as Initial Monotherapy Treatment
Adverse Reactions Leading to Discontinuation in Controlled Clinical Studies
In the double-blind, placebo-controlled trials conducted in patients receiving AZILECT as monotherapy, approximately 5% of the 149 patients treated with rasagiline discontinued treatment due to adverse reactions compared to 2% of the 151 patients who received placebo.
The only adverse reaction that led to the discontinuation of more than one patient was hallucinations.
Adverse Reaction Incidence in Controlled Clinical Studies
The most commonly observed adverse reactions were those in which the treatment difference for the incidence in AZILECT-treated patients was ≥ 3 % greater than the incidence in the placebo-treated patients and included flu syndrome, arthralgia, depression, and dyspepsia. Table 1 lists treatment-emergent adverse reactions that occurred in ≥ 2% of patients receiving AZILECT as monotherapy participating in the double-blind, placebo-controlled trial and were numerically more frequent than in the placebo group.
| Placebo-Controlled Studies Without Levodopa Treatment | AZILECT 1 mg (N=149) |
Placebo (N=151) |
|---|---|---|
|
|
% of Patients | % of Patients |
| Headache | 14 | 12 |
| Arthralgia | 7 | 4 |
| Dyspepsia | 7 | 4 |
| Depression | 5 | 2 |
| Fall | 5 | 3 |
| Flu syndrome | 5 | 1 |
| Conjunctivitis | 3 | 1 |
| Fever | 3 | 1 |
| Gastroenteritis | 3 | 1 |
| Rhinitis | 3 | 1 |
| Arthritis | 2 | 1 |
| Ecchymosis | 2 | 0 |
| Malaise | 2 | 0 |
| Neck Pain | 2 | 0 |
| Paresthesia | 2 | 1 |
| Vertigo | 2 | 1 |
Other events of potential clinical importance reported by 1% or more of patients receiving AZILECT as monotherapy, and at least as frequent as in the placebo group, in descending order of frequency include: dizziness, diarrhea, chest pain, albuminuria, allergic reaction, alopecia, angina pectoris, anorexia, asthma, hallucinations, impotence, leukopenia, libido decreased, liver function tests abnormal, skin carcinoma, syncope, vesiculobullous rash, vomiting.
There were no significant differences in the safety profile based on age or gender.
Patients Receiving AZILECT as Adjunct to Levodopa Therapy
Adverse Reactions Leading to Discontinuation in Controlled Clinical Studies
In a double-blind, placebo-controlled trial (Study 1) conducted in patients treated with AZILECT as adjunct to levodopa therapy, approximately 9% of the 164 patients treated with AZILECT 0.5 mg/day and 7% of the 149 patients treated with AZILECT 1 mg/day discontinued treatment due to adverse reactions compared to 6% of the 159 patients who received placebo. The adverse reactions that led to discontinuation of more than one rasagiline-treated patient were: diarrhea, weight loss, hallucination, and rash. Adverse event reporting was considered more reliable for Study 1 than for the second controlled trial (Study 2); therefore only the adverse event data from Study 1 are presented in this section of labeling.
Adverse Reactions: Incidence in Controlled Clinical Studies
The most commonly observed adverse reactions were those in which the treatment difference for the incidence in AZILECT-treated patients (n=149) was ≥ 3 % greater than the incidence in the placebo-treated patients (n=159) and included dyskinesia, accidental injury, weight loss, postural hypotension, vomiting, anorexia, arthralgia, abdominal pain, nausea, constipation, dry mouth, rash, abnormal dreams, and fall.
Table 2 lists treatment-emergent adverse reactions that occurred in ≥ 2% of patients treated with AZILECT 1 mg/day as adjunct to levodopa therapy participating in the double-blind, placebo-controlled trial (Study 1) and that were numerically more frequent than the placebo group. The table also shows the rates for the 0.5 mg group in Study 1.
|
|
AZILECT 1 mg + Levodopa (N=149) |
AZILECT 0.5 mg + Levodopa (N=164) |
Placebo + Levodopa (N=159) |
|---|---|---|---|
|
|
% of patients | % of patients | % of patients |
| Dyskinesia | 18 | 18 | 10 |
| Accidental injury | 12 | 8 | 5 |
| Nausea | 12 | 10 | 8 |
| Headache | 11 | 8 | 10 |
| Fall | 11 | 12 | 8 |
| Weight loss | 9 | 2 | 3 |
| Constipation | 9 | 4 | 5 |
| Postural hypotension | 9 | 6 | 3 |
| Arthralgia | 8 | 6 | 4 |
| Vomiting | 7 | 4 | 1 |
| Dry mouth | 6 | 2 | 3 |
| Rash | 6 | 3 | 3 |
| Somnolence | 6 | 4 | 4 |
| Abdominal pain | 5 | 2 | 1 |
| Anorexia | 5 | 2 | 1 |
| Diarrhea | 5 | 7 | 4 |
| Ecchymosis | 5 | 2 | 3 |
| Dyspepsia | 5 | 4 | 4 |
| Paresthesia | 5 | 2 | 3 |
| Abnormal dreams | 4 | 1 | 1 |
| Hallucinations | 4 | 5 | 3 |
| Ataxia | 3 | 6 | 1 |
| Dyspnea | 3 | 5 | 2 |
| Infection | 3 | 2 | 2 |
| Neck pain | 3 | 1 | 1 |
| Sweating | 3 | 2 | 1 |
| Tenosynovitis | 3 | 1 | 0 |
| Dystonia | 3 | 2 | 1 |
| Gingivitis | 2 | 1 | 1 |
| Hemorrhage | 2 | 1 | 1 |
| Hernia | 2 | 1 | 1 |
| Myasthenia | 2 | 2 | 1 |
Several of the more common adverse reactions seemed dose-related, including weight loss, postural hypotension, and dry mouth.
Other adverse reactions of potential clinical importance reported in Study 1 by 1% or more of patients treated with rasagiline 1 mg/day as adjunct to levodopa therapy, and at least as frequent as in the placebo group, in descending order of frequency include : skin carcinoma, anemia, albuminuria, amnesia, arthritis, bursitis, cerebrovascular accident, confusion, dysphagia, epistaxis, leg cramps, pruritus, skin ulcer.
There were no significant differences in the safety profile based on age or gender.
Other Adverse Reactions Observed During All Phase 2/3 Clinical Trials
Rasagiline was administered to approximately 1361 patients during all PD phase 2/3 clinical trials. About 283 patients received rasagiline for at least one year, approximately 410 patients received rasagiline for at least two years, 116 patients received rasagiline for at least 3 years, and 245 patients received rasagiline for more than 3 years, with some patients treated for more than 5 years. The long-term safety profile was similar to that observed with shorter duration exposure.
The frequencies listed below represent the proportion of the 1361 individuals exposed to rasagiline who experienced events of the type cited.
All events that occurred at least twice (or once for serious or potentially serious events), except those already listed above, trivial events, terms too vague to be meaningful, adverse events with no plausible relation to treatment, and events that would be expected in patients of the age studied, were reported without regard to determination of a causal relationship to rasagiline.
Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in at least 1/100 patients, infrequent adverse events are defined as those occurring in at least 1/100 to 1/1000 patients and rare adverse events are defined as those occurring in fewer than 1/1000 patients.
Body as a whole: Frequent: asthenia Infrequent: chills, face edema, flank pain, photosensitivity reaction
Cardiovascular system: Frequent: bundle branch block Infrequent: deep thrombophlebitis, heart failure, migraine, myocardial infarct, phlebitis, ventricular tachycardia Rare: arterial thrombosis, atrial arrhythmia, AV block complete, AV block second degree, bigeminy, cerebral hemorrhage, cerebral ischemia, ventricular fibrillation
Digestive system: Frequent: gastrointestinal hemorrhage Infrequent: colitis, esophageal ulcer, esophagitis, fecal incontinence, intestinal obstruction, mouth ulceration, stomach ulcer, stomatitis, tongue edema Rare: hematemesis, hemorrhagic gastritis, intestinal perforation, intestinal stenosis, jaundice, large intestine perforation, megacolon, melena
Hemic and Lymphatic system: Infrequent: macrocytic anemia Rare: purpura, thrombocythemia
Metabolic and Nutritional disorders: Infrequent: hypocalcemia
Musculoskeletal system: Infrequent: bone necrosis, muscle atrophy Rare: arthrosis
Nervous system: Frequent: abnormal gait, anxiety, hyperkinesia, hypertonia, neuropathy, tremor Infrequent: agitation, aphasia, circumoral paresthesia, convulsion, delusions, dementia, dysarthria, dysautonomia, dysesthesia, emotional lability, facial paralysis, foot drop, hemiplegia, hypesthesia, incoordination, manic reaction, myoclonus, neuritis, neurosis, paranoid reaction, personality disorder, psychosis, wrist drop Rare: apathy, delirium, hostility, manic depressive reaction, myelitis, neuralgia, psychotic depression, stupor
Respiratory system: Frequent: cough increased Infrequent: apnea, emphysema, laryngismus, pleural effusion, pneumothorax Rare: interstitial pneumonia, larynx edema, lung fibrosis
Skin and Appendages: Infrequent: eczema, urticaria Rare: exfoliative dermatitis, leukoderma
Special senses: Infrequent: blepharitis, deafness, diplopia, eye hemorrhage, eye pain, glaucoma, keratitis, ptosis, retinal degeneration, taste perversion, visual field defect Rare: blindness, parosmia, photophobia, retinal detachment, retinal hemorrhage, strabismus, taste loss, vestibular disorder
Urogenital system: Frequent: hematuria, urinary incontinence Infrequent: acute kidney failure, dysmenorrhea, dysuria, kidney calculus, nocturia, polyuria, scrotal edema, sexual function abnormal, urinary retention, urination impaired, vaginal hemorrhage, vaginal moniliasis, vaginitis Rare: abnormal ejaculation, amenorrhea, anuria, epididymitis, gynecomastia, hydroureter, leukorrhea, priapism
6.2 Post-marketing Experience
The following adverse events not described in sections 4 and 5 have been identified during the post-marketing/post-approval use of AZILECT. Because these adverse events are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency nor to establish unequivocally a causal relationship to drug exposure: Increased libido including hypersexuality, impulse control symptoms, pathological gambling [see Patient Counseling Information (17.11)]
7 DRUG INTERACTIONS
Enter section text here
7.1 Meperidine
Serious, sometimes fatal reactions have been precipitated with concomitant use of meperidine (e.g., Demerol and other tradenames) and MAO inhibitors including selective MAO-B inhibitors [see Contraindications (4.1)].
7.2 Dextromethorphan
The concomitant use of AZILECT and dextromethorphan was not allowed in clinical studies. The combination of MAO inhibitors and dextromethorphan has been reported to cause brief episodes of psychosis or bizarre behavior. Therefore, in view of AZILECT's MAO inhibitory activity, dextromethorphan should not be used concomitantly with AZILECT [see Contraindications (4.2)].
7.3 Sympathomimetic Medications
The concomitant use of AZILECT and sympathomimetic medications was not allowed in clinical studies. Severe hypertensive reactions have followed the administration of sympathomimetics and non-selective MAO inhibitors. One case of hypertensive crisis has been reported in a patient taking the recommended dose of a selective MAO-B inhibitor and a sympathomimetic medication (ephedrine). Elevated blood pressure was reported in another patient taking the recommended dose of AZILECT and ophthalmic drops with a sympathomimetic medication (tetrahydrozoline).
Because AZILECT is a selective MAOI, hypertensive reactions are not ordinarily expected with the concomitant use of sympathomimetic medications. Nevertheless, caution should be exercised when concomitantly using recommended doses of AZILECT with any sympathomimetic medications including nasal, oral, and ophthalmic decongestants and cold remedies.
7.4 MAO Inhibitors
AZILECT should not be administered along with other MAO inhibitors because of the increased risk of non-selective MAO inhibition that may lead to a hypertensive crisis [see Contraindications (4.3)].
7.5 Antidepressants
Concomitant use of AZILECT with one of many classes of antidepressants (e.g., SSRIs, SNRIs, triazolopyridine, tricyclic or tetracyclic antidepressants) is not recommended [see Warnings and Precautions (5.1)].
7.6 Levodopa/Carbidopa
[see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
7.7 Ciprofloxacin and Other CYP1A2 Inhibitors
Rasagiline plasma concentrations may increase up to 2 fold in patients using concomitant ciprofloxacin and other CYP1A2 inhibitors. This could result in increased adverse events [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)].
7.8 Theophylline
[see Clinical Pharmacology (12.3)].
7.9 Tyramine/Rasagiline Interaction
MAO in the gastrointestinal tract and liver (primarily type A) is thought to provide vital protection from exogenous amines (e.g., tyramine) that have the capacity, if absorbed intact, to cause a "hypertensive crisis," the so-called "cheese reaction". If large amounts of certain exogenous amines (e.g., from fermented cheese, herring, over-the-counter cough/cold medications) gain access to the systemic circulation because MAO-A has been inhibited, they cause release of norepinephrine which may result in a rise in systemic blood pressure. MAOIs that selectively inhibit MAO-B are largely devoid of the potential to cause tyramine-induced hypertensive crisis.
Results of a special tyramine challenge study indicate that rasagiline is selective for MAO-B at recommended doses and can ordinarily be used without dietary tyramine restriction. However, certain foods (e.g., aged cheeses, such as Stilton cheese) may contain very high amounts (i.e., > 150 mg) of tyramine and could potentially cause a hypertensive cheese reaction in patients taking AZILECT due to mild increased sensitivity to tyramine. Patients should be advised to avoid foods (e.g., aged cheese) containing a very large amount of tyramine while taking recommended doses of AZILECT because of the potential for large increases in blood pressure. Selectivity for inhibiting MAO-B diminishes in a dose-related manner as the dose is progressively increased above the recommended daily doses.
There were no cases of hypertensive crisis in the clinical development program associated with 1 mg daily rasagiline treatment, in which most patients did not follow dietary tyramine restriction.
Despite the selective inhibition of MAO-B at recommended doses of AZILECT, there have been post-marketing reports of patients who experienced significantly elevated blood pressure (including rare cases of hypertensive crisis) after ingestion of unknown amounts of tyramine-rich foods while taking recommended doses of AZILECT [see Dosing and Administration (2), and Warnings and Precautions (5.4)].
8 USE IN SPECIFIC POPULATIONS
Enter section text here
8.1 Pregnancy
Category C
No effect on embryo-fetal development was observed in a combined mating/fertility and embryo-fetal development study in female rats at doses up to 3 mg/kg/day (approximately 30 times the expected plasma rasagiline exposure (AUC) at the maximum recommended human dose [MRHD, 1 mg/day]). Effects on embryo-fetal development in rabbit have not been adequately assessed.
In a study in which pregnant rats were dosed with rasagiline (0.1, 0.3, 1 mg/kg/day) orally, from the beginning of organogenesis to day 20 post-partum, offspring survival was decreased and offspring body weight was reduced at doses of 0.3 mg/kg/day and 1 mg/kg/day (10 and 16 times the expected plasma rasagiline exposure [AUC] at the MRHD). No plasma data were available at the no-effect dose (0.1 mg/kg); however, that dose is 1 times the MRHD on a mg/m2 basis. Rasagiline's effect on physical and behavioral development was not adequately assessed in this study.
Rasagiline may be given as an adjunct therapy to levodopa/carbidopa treatment. In a study in which pregnant rats were dosed with rasagiline (0.1, 0.3, 1 mg/kg/day) and levodopa/carbidopa (80/20 mg/kg/day) (alone and in combination) throughout the period of organogenesis, there was an increased incidence of wavy ribs in fetuses from rats treated with rasagiline in combination with levodopa/carbidopa at 1/80/20 mg/kg/day (approximately 8 times the plasma AUC expected in humans at the MRHD and 1/1 times the MRHD of levodopa/carbidopa [800/200 mg/day] on a mg/m2 basis). In a study in which pregnant rabbits were dosed throughout the period of organogenesis with rasagiline alone (3 mg/kg) or in combination with levodopa/carbidopa (rasagiline: 0.1, 0.6, 1.2 mg/kg, levodopa/carbidopa: 80/20 mg/kg/day), an increase in embryo-fetal death was noted at rasagiline doses of 0.6 and 1.2 mg/kg/day when administered in combination with levodopa/carbidopa (approximately 7 and 13 times, respectively, the plasma rasagiline AUC at the MRHD). There was an increase in cardiovascular abnormalities with levodopa/carbidopa alone (1/1 times the MRHD on a mg/m2 basis) and to a greater extent when rasagiline (at all doses; 1-13 times the plasma rasagiline AUC at the MRHD) was administered in combination with levodopa/carbidopa.
There are no adequate and well-controlled studies of rasagiline in pregnant women. Therefore, AZILECT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
In rats rasagiline was shown to inhibit prolactin secretion and it may inhibit milk secretion in females.
It is not known whether rasagiline is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when AZILECT is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of AZILECT in the pediatric population have not been studied.
8.5 Geriatric Use
Approximately half of patients in clinical trials were 65 years and over. There were no significant differences in the safety profile of the geriatric and non-geriatric patients.
8.6 Hepatic Impairment
Rasagiline plasma concentration may be increased in patients with
mild (up to 2 fold, Child-Pugh score 5-6), moderate (up to 7 fold, Child-Pugh
score 7-9), and severe (Child-Pugh score 10-15) hepatic impairment. Patients
with mild hepatic impairment should be given the dose of 0.5 mg/day. AZILECT
should not be used in patients with moderate or severe hepatic impairment [see Dosage and Administration (2.3), Warnings and Precautions (5.3) and Clinical
Pharmacology (12.3)].
8.7 Renal Impairment
Dose adjustment of AZILECT is not required for patients with mild or moderate renal impairment because AZILECT plasma concentrations are not increased in patients with moderate renal impairment. Rasagiline has not been studied in patients with severe renal impairment.
9 DRUG ABUSE AND DEPENDENCE
Enter section text here
9.1 Controlled Substance
AZILECT is not a controlled substance.
9.2 Abuse
Studies conducted in mice and rats did not reveal any potential for drug abuse and dependence. Clinical trials have not revealed any evidence of the potential for abuse, tolerance or physical dependence; however, systematic studies in humans designed to evaluate these effects have not been performed.
9.3 Dependence
Studies conducted in mice and rats did not reveal any potential for drug abuse and dependence. Clinical trials have not revealed any evidence of the potential for abuse, tolerance or physical dependence; however, systematic studies in humans designed to evaluate these effects have not been performed.
10 OVERDOSAGE
No cases of AZILECT overdose were reported in clinical trials.
Rasagiline was well tolerated in a single-dose study in healthy volunteers receiving 20 mg/day and in a ten-day study in healthy volunteers receiving 10 mg/day. Adverse events were mild or moderate. In a dose escalation study in patients on chronic levodopa therapy treated with 10 mg of rasagiline there were three reports of cardiovascular side effects (including hypertension and postural hypotension) which resolved following treatment discontinuation.
Symptoms of overdosage, although not observed with rasagiline during clinical development, may resemble those observed with non-selective MAO inhibitors (MAOIs).
Although no cases of overdose have been observed with rasagiline during the clinical development program, the following description of presenting symptoms and clinical course is based upon overdose descriptions of non-selective MAO inhibitors.
Characteristically, signs and symptoms of non-selective MAOI overdose may not appear immediately. Delays of up to 12 hours between ingestion of drug and the appearance of signs may occur. Importantly, the peak intensity of the syndrome may not be reached for upwards of a day following the overdose. Death has been reported following overdosage. Therefore, immediate hospitalization, with continuous patient observation and monitoring for a period of at least two days following the ingestion of such drugs in overdose, is strongly recommended.
The clinical picture of MAOI overdose varies considerably; its severity may be a function of the amount of drug consumed. The central nervous and cardiovascular systems are prominently involved.
Signs and symptoms of overdosage may include, alone or in combination, any of the following: drowsiness, dizziness, faintness, irritability, hyperactivity, agitation, severe headache, hallucinations, trismus, opisthotonos, convulsions, and coma; rapid and irregular pulse, hypertension, hypotension and vascular collapse; precordial pain, respiratory depression and failure, hyperpyrexia, diaphoresis, and cool, clammy skin.
There is no specific antidote for rasagiline overdose. The following suggestions are offered based upon the assumption that rasagiline overdose may be modeled after non-selective MAO inhibitor poisoning. Treatment of overdose with non-selective MAO inhibitors is symptomatic and supportive. Respiration should be supported by appropriate measures, including management of the airway, use of supplemental oxygen, and mechanical ventilatory assistance, as required. Body temperature should be monitored closely. Intensive management of hyperpyrexia may be required. Maintenance of fluid and electrolyte balance is essential. For this reason, in cases of overdose with AZILECT, dietary tyramine restriction should be observed for several weeks to avoid the risk of a hypertensive/cheese reaction.
A poison control center should be called for the most current treatment guidelines.
A post-marketing report described a single patient who developed a non-fatal serotonin syndrome after ingesting 100 mg of AZILECT in a suicide attempt. Another patient who was treated in error with 4 mg AZILECT daily and tramadol also developed a serotonin syndrome. One patient who was treated in error with 3 mg AZILECT daily experienced alternating episodes of vascular fluctuations consisting of hypertension and orthostatic hypotension.
11 DESCRIPTION
AZILECT® tablets contain rasagiline (as the mesylate), a propargylamine-based drug indicated for the treatment of idiopathic Parkinson's disease. It is designated chemically as: 1H-Inden-1-amine, 2, 3-dihydro-N-2-propynyl-, (1R)-, methanesulfonate. The empirical formula of rasagiline mesylate is (C12H13N)CH4SO3 and its molecular weight is 267.34.
Its structural formula is:
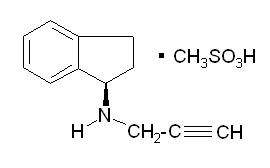
Rasagiline mesylate is a white to off-white powder, freely soluble in water or ethanol and sparingly soluble in isopropanol. Each AZILECT tablet for oral administration contains rasagiline mesylate equivalent to 0.5 mg or 1 mg of rasagiline base.
Each AZILECT tablet also contains the following inactive ingredients: mannitol, starch, pregelatinized starch, colloidal silicon dioxide, stearic acid and talc.
12 CLINICAL PHARMACOLOGY
Enter section text here
12.1 Mechanism of Action
AZILECT functions as a selective, irreversible MAO-B inhibitor indicated for the treatment of idiopathic Parkinson's disease. The results of a clinical trial designed to examine the effects of Azilect on blood pressure when it is administered with increasing doses of tyramine indicates the functional selectivity can be incomplete when healthy subjects ingest large amounts of tyramine while receiving recommended doses of AZILECT. The selectivity for inhibiting MAO-B diminishes in a dose-related manner.
MAO, a flavin-containing enzyme, is classified into two major molecular species, A and B, and is localized in mitochondrial membranes throughout the body in nerve terminals, brain, liver and intestinal mucosa. MAO regulates the metabolic degradation of catecholamines and serotonin in the CNS and peripheral tissues. MAO-B is the major form in the human brain. In ex vivo animal studies in brain, liver and intestinal tissues, rasagiline was shown to be a potent, irreversible monoamine oxidase type B (MAO-B) selective inhibitor. Rasagiline at the recommended therapeutic dose was also shown to be a potent and irreversible inhibitor of MAO-B in platelets. The precise mechanisms of action of rasagiline are unknown. One mechanism is believed to be related to its MAO-B inhibitory activity, which causes an increase in extracellular levels of dopamine in the striatum. The elevated dopamine level and subsequent increased dopaminergic activity are likely to mediate rasagiline's beneficial effects seen in models of dopaminergic motor dysfunction.
12.2 Pharmacodynamics
Platelet MAO Activity in Clinical Studies
Studies in healthy subjects and in Parkinson's disease patients have shown that rasagiline inhibits platelet MAO-B irreversibly. The inhibition lasts at least 1 week after last dose. Almost 25-35% MAO-B inhibition was achieved after a single rasagiline dose of 1 mg/day and more than 55% of MAO-B inhibition was achieved after a single rasagiline dose of 2 mg/day. Over 90% inhibition was achieved 3 days after rasagiline daily dosing at 2 mg/day and this inhibition level was maintained 3 days post-dose. Multiple doses of rasagiline of 0.5, 1 and 2 mg per day resulted in complete MAO-B inhibition.
12.3 Pharmacokinetics
Rasagiline in the range of 1-6 mg demonstrated a more than proportional increase in AUC, while Cmax was dose proportional. Rasagiline mean steady-state half life is 3 hours but there is no correlation of pharmacokinetics with its pharmacological effect because of its irreversible inhibition of MAO-B.
Absorption
Rasagiline is rapidly absorbed, reaching peak plasma concentration (Cmax) in approximately 1 hour. The absolute bioavailability of rasagiline is about 36%.
Food does not affect the Tmax of rasagiline, although Cmax and exposure (AUC) are decreased by approximately 60% and 20%, respectively, when the drug is taken with a high fat meal. Because AUC is not significantly affected, AZILECT can be administered with or without food [see Dosage and Administration (2)].
Distribution
The mean volume of distribution at steady-state is 87 L, indicating that the tissue binding of rasagiline is in excess of plasma protein binding. Plasma protein binding ranges from 88-94% with mean extent of binding of 61-63% to human albumin over the concentration range of 1-100 ng/mL.
Metabolism and Elimination
Rasagiline undergoes almost complete biotransformation in the liver prior to excretion. The metabolism of rasagiline proceeds through two main pathways: N-dealkylation and/or hydroxylation to yield 1-aminoindan (AI), 3-hydroxy-N-propargyl-1 aminoindan (3-OH-PAI) and 3-hydroxy-1-aminoindan (3-OH-AI). In vitro experiments indicate that both routes of rasagiline metabolism are dependent on the cytochrome P450 (CYP) system, with CYP1A2 being the major isoenzyme involved in rasagiline metabolism. Glucuronide conjugation of rasagiline and its metabolites, with subsequent urinary excretion, is the major elimination pathway.
After oral administration of 14C-labeled rasagiline, elimination occurred primarily via urine and secondarily via feces (62% of total dose in urine and 7% of total dose in feces over 7 days), with a total calculated recovery of 84% of the dose over a period of 38 days. Less than 1% of rasagiline was excreted as unchanged drug in urine.
Special Populations
Hepatic Impairment
Following repeat dose administration (7 days) of rasagiline (1 mg/day) in subjects with mild hepatic impairment (Child-Pugh score 5-6), AUC and Cmax were increased by 2 fold and 1.4 fold, respectively, compared to healthy subjects. In subjects with moderate hepatic impairment (Child-Pugh score 7-9), AUC and Cmax were increased by 7 fold and 2 fold, respectively, compared to healthy subjects [see Dosage and Administration (2.3) and Warnings and Precautions (5.3)].
Renal Impairment
Following repeat dose administration (8 days) of rasagiline (1 mg/day) in subjects with moderate renal impairment, rasagiline exposure (AUC) was similar to rasagiline exposure in healthy subjects, while the major metabolite 1-AI exposure (AUC) was increased 1.5- fold in subjects with moderate renal impairment, compared to healthy subjects. Because 1-AI is not an MAO inhibitor, no dose adjustment is needed for patients with mild and moderate renal impairment. Data are not available for patients with severe renal impairment.
Elderly
Since age has little influence on rasagiline pharmacokinetics, it can be administered at the recommended dose in the elderly (≥ 65 years).
Pediatric
AZILECT has not been investigated in patients below 18 years of age.
Gender
The pharmacokinetic profile of rasagiline is similar in men and women.
Drug-Drug Interactions
Tyramine Effect
[see Dosage and Administration (2), Warnings and Precautions (5.4), and Drug Interactions (7.9)].
Levodopa
Data from population pharmacokinetic studies comparing rasagiline clearance in the presence and absence of levodopa have given conflicting results. Although there may be some increase in rasagiline blood levels in the presence of levodopa, the effect is modest and rasagiline dosing need not be modified in the presence of levodopa.
Effect of Other Drugs on the Metabolism of AZILECT
In vitro metabolism studies showed that CYP1A2 was the major enzyme responsible for the metabolism of rasagiline. There is the potential for inhibitors of this enzyme to alter AZILECT clearance when coadministered [see Dosage and Administration (2.5) and Warnings and Precautions (5.2)].
Ciprofloxacin: When ciprofloxacin, an inhibitor of CYP1A2, was administered to healthy volunteers (n=12) at 500 mg (BID) with rasagiline at 2 mg/day, the AUC of rasagiline increased by 83% and there was no change in the elimination half life [see Dosage and Administration (2.5) and Warnings and Precautions (5.2)].
Theophylline: Coadministration of rasagiline 1 mg/day and theophylline, a substrate of CYP1A2, up to 500 mg twice daily to healthy subjects (n=24) did not affect the pharmacokinetics of either drug.
Antidepressants: Severe CNS toxicity (occasionally fatal) associated with hyperpyrexia as part of a serotonin syndrome, has been reported with combined treatment of an antidepressant (e.g., from one of many classes including tricyclic or tetracyclic antidepressants, SSRIs, SNRIs, triazolopyridine antidepressants) and non-selective MAOI or a selective MAO-B inhibitor [see Warnings and Precautions (5.1)].
Effect of AZILECT on Other Drugs
No additional in vivo trials have investigated the effect of AZILECT on other drugs metabolized by the cytochrome P450 enzyme system. In vitro studies showed that rasagiline at a concentration of 1mcg/ml (equivalent to a level that is 160 times the average Cmax ~ 5.9-8.5 ng/mL in Parkinson's disease patients after 1 mg rasagiline multiple dosing) did not inhibit cytochrome P450 isoenzymes, CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4 and CYP4A. These results indicate that rasagiline is unlikely to cause any clinically significant interference with substrates of these enzymes.
13 NONCLINICAL TOXICOLOGY
Enter section text here
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Two year carcinogenicity studies were conducted in CD-1 mice at oral (gavage) doses of 1, 15, and 45 mg/kg and in Sprague-Dawley rats at oral (gavage) doses of 0.3, 1, and 3 mg/kg (males) or 0.5, 2, 5, and 17 mg/kg (females). In rats, there was no increase in tumors at any dose tested. Plasma exposures at the highest dose tested were approximately 33 and 260 times, in male and female rats, respectively, the expected plasma exposures in humans at the maximum recommended dose (MRD) of 1 mg/day.
In mice, there was an increase in lung tumors (combined adenomas/carcinomas) at 15 and 45 mg/kg males and females. Plasma exposures associated with the no-effect dose (1 mg/kg) were approximately 5 times those expected in humans at the MRD.
The carcinogenic potential of rasagiline administered in combination with levodopa/carbidopa has not been examined.
Mutagenesis
Rasagiline was reproducibly clastogenic in in vitro chromosomal aberration assays in human lymphocytes in the presence of metabolic activation and was mutagenic and clastogenic in the in vitro mouse lymphoma tk assay in the absence and presence of metabolic activation. Rasagiline was negative in the in vitro bacterial reverse mutation (Ames) assay, the in vivo unscheduled DNA synthesis assay, and the in vivo micronucleus assay in CD-1 mice. Rasagiline was also negative in the in vivo micronucleus assay in CD-1 mice when administered in combination with levodopa/carbidopa.
Impairment of Fertility
Rasagiline had no effect on mating performance or fertility in male rats treated prior to and throughout the mating period, or in female rats treated from prior to mating through day 17 of gestation at oral doses up to 3 mg/kg/day (approximately 30 times the expected plasma rasagiline exposure (AUC) at the maximum recommended human dose [1 mg/day]). The effect of rasagiline administered in combination with levodopa/carbidopa on mating and fertility has not been examined.
14 CLINICAL TRIALS
The effectiveness of AZILECT for the treatment of Parkinson's disease was established in three 18- to 26-week, randomized, placebo-controlled trials. In one of these trials AZILECT was given as initial monotherapy and in the other two as adjunctive therapy to levodopa.
14.1 Monotherapy Use of AZILECT
The monotherapy trial was a double-blind, randomized, fixed-dose parallel group, 26-week study in early Parkinson's disease patients not receiving any concomitant dopaminergic therapy at the start of the study. The majority of the patients were not treated with any anti-Parkinson's disease medication before receiving rasagiline treatment.
In this trial, 404 patients were randomly assigned to receive placebo (138 patients), rasagiline 1 mg/day (134 patients) or rasagiline 2 mg/day (132 patients). Patients were not allowed to take levodopa, dopamine agonists, selegiline or amantadine, but if necessary, could take stable doses of anticholinergic medication. The average Parkinson's disease duration was approximately 1 year (range 0 to 11 years).
The primary measure of effectiveness was the change from baseline in the total score of the Unified Parkinson's Disease Rating Scale (UPDRS), [mentation (Part I) + activities of daily living (ADL) (Part II) + motor function (Part III)]. The UPDRS is a multi-item rating scale that measures the ability of a patient to perform mental and motor tasks as well as activities of daily living. A reduction in the score represents improvement and a beneficial change from baseline appears as a negative number.
Rasagiline (1 or 2 mg once daily) had a significant beneficial effect relative to placebo on the primary measure of effectiveness in patients receiving six months of treatment and not on dopaminergic therapy. Patients who received rasagiline had significantly less worsening in the UPDRS score, compared to those who received placebo. The effectiveness of rasagiline 1 mg and 2 mg was comparable. Table 3 displays the results of the monotherapy trial.
| Primary Measure of Effectiveness: Change in total UPDRS score | |||
|---|---|---|---|
|
|
Baseline score | Change from baseline to termination score | p-value vs. placebo |
| Placebo | 24.5 | 3.9 | --- |
| 1.0 mg/day | 24.7 | 0.1 | 0.0001 |
| 2.0 mg/day | 25.9 | 0.7 | 0.0001 |
For the comparison between rasagiline 1 mg/day and placebo, no differences in effectiveness based on age or gender were detected.
14.2 Adjunctive use of AZILECT
Two multicenter, randomized, multinational trials were conducted in more advanced Parkinson's disease patients treated chronically with levodopa and experiencing motor fluctuations (including but not limited to, end of dose "wearing off," sudden or random "off," etc.). The first (Study 1) was conducted in North America (U.S. and Canada) and compared two doses (0.5 mg and 1 mg daily) of rasagiline and placebo while the second (Study 2) was conducted outside of North America (several European countries, Argentina, Israel) and studied only a single dose (1 mg daily) of rasagiline and placebo. Patients had had Parkinson's disease for an average of 9 years (range 5 months to 33 years), had been taking levodopa for an average of 8 years (range 5 months to 32 years), and had been experiencing motor fluctuations for approximately 3 to 4 years (range 1 month to 23 years). Patients kept home diaries just prior to baseline and at specified intervals during the trial. Diaries recorded one of the following four conditions for each half-hour interval over a 24-hour period: "ON" (period of relatively good function and mobility) as either "ON" with no dyskinesia or without troublesome dyskinesia, or "ON" with troublesome dyskinesia, "OFF" (period of relatively poor function and mobility) or asleep. "Troublesome" dyskinesia is defined as that which interferes with the patient's daily activity. All patients had been inadequately controlled and were experiencing motor fluctuations typical of advanced stage disease despite receiving levodopa/decarboxylase inhibitor. The average dose of levodopa/decarboxylase inhibitor was approximately 700 to 800 mg (range 150 to 3000 mg/day). Patients were also allowed to take stable doses of additional anti-PD medications at entry into the trials. In both trials, approximately 65% of patients were on dopamine agonists and in the North American study (Study 1) approximately 35% were on entacapone. The majority of patients taking entacapone were taking a dopamine agonist as well.
In both trials the primary measure of effectiveness was the change in the mean number of hours that were spent in the "OFF" state at baseline compared to the mean number of hours that were spent in the "OFF" state during the treatment period.
The first adjunct study (Study 1) was a double-blind, randomized, fixed-dose, parallel group trial conducted in 472 levodopa-treated Parkinson's disease patients who were experiencing motor fluctuations. Patients were randomly assigned to receive placebo (159 patients), rasagiline 0.5 mg/day (164 patients), or rasagiline 1 mg/day (149 patients), and were treated for 26 weeks. Patients averaged approximately 6 hours daily in the "OFF" state at baseline, as confirmed by home diaries.
The second adjunct study (Study 2) was a double-blind, randomized, parallel group trial conducted in 687 levodopa-treated Parkinson's disease patients who were experiencing motor fluctuations. Patients were randomly assigned to receive placebo (229 patients), rasagiline 1 mg/day (231 patients) or an active comparator, a COMT inhibitor taken along with scheduled doses of levodopa/decarboxylase inhibitor (227 patients). Patients were treated for 18 weeks. Patients averaged approximately 5.6 hours daily in the "OFF" state at baseline as confirmed by home diaries.
In both studies, rasagiline 1 mg once daily reduced "OFF" time compared to placebo when added to levodopa in patients experiencing motor fluctuations (Tables 4 and 5). The lower dose (0.5 mg) of rasagiline also significantly reduced "OFF" time (Table 4), but had a numerically smaller effect than the 1 mg dose of rasagiline. In Study 2, the active comparator also reduced "OFF" time when compared to placebo.
|
Primary Measure of Effectiveness: Change in mean total daily "OFF" time |
|
|
|
|
|
Baseline (hours) |
Change from baseline to treatment period (hours) |
p-value vs. placebo |
| Placebo |
6.0 |
-0.9 |
--- |
| 0.5 mg/day |
6.0 |
-1.4 |
0.0199 |
| 1.0 mg/day |
6.3 |
-1.9 |
<0.0001 |
|
Primary Measure of Effectiveness: Change in mean total daily "OFF" time |
|
|
|
|
|
Baseline (hours) |
Change from baseline to treatment period (hours) |
p-value vs. placebo |
| Placebo |
5.5 |
-0.40 |
--- |
| 1.0 mg/day |
5.6 |
-1.2 |
0.0001 |
In both studies, dosage reduction of levodopa was allowed within the first 6 weeks if dopaminergic side effects, including dyskinesia and hallucinations, emerged. In Study 1, levodopa dosage reduction occurred in 8% of patients in the placebo group and in 16% and 17% of patients in the 0.5 mg/day and 1 mg/day rasagiline groups, respectively. In those patients who had levodopa dosage reduced, the dose was reduced on average by about 7%, 9%, and 13% in the placebo, 0.5 mg/day, and 1 mg/day groups, respectively. In Study 2, levodopa dosage reduction occurred in 6% of patients in the placebo group and in 9% in the rasagiline 1 mg/day group. In patients who had their levodopa dosage reduced, the dose was reduced on average by about 13% and 11% in the placebo and the rasagiline groups, respectively.
For the comparison between rasagiline 1 mg/day and placebo in both studies, no differences in effectiveness based on age or gender were detected.
Several secondary outcome assessments in the two studies showed statistically significant improvements with rasagiline. These included effects on the activities of daily living (ADL) subscale of the UPDRS performed during an "OFF" period and the motor subscale of the UPDRS performed during an "ON" period. In both scales, a negative response represents improvement. Tables 6 and 7 show these results for Studies 1 and 2.
|
|
Baseline (score) |
Change from baseline to last value
|
|
UPDRS ADL (Activities of Daily Living) subscale score while "OFF" |
|
|
| Placebo |
15.5 |
0.68 |
| 0.5 mg/day |
15.8 |
-0.60 |
| 1.0 mg/day |
15.5 |
-0.68 |
| UPDRS Motor subscale score while "ON" |
|
|
| Placebo |
20.8 |
1.21 |
| 0.5 mg/day |
21.5 |
-1.43 |
| 1.0 mg/day |
20.9 |
1/30 |
|
|
Baseline (score)
|
Change from baseline to last value |
|
UPDRS ADL (Activities of Daily Living) subscale score while "OFF" |
|
|
| Placebo |
18.7 |
-0.89 |
| 1.0 mg/day |
19.0 |
-2.61 |
| UPDRS Motor subscale score while "ON" |
|
|
| Placebo |
23.5 |
-0.82 |
| 1.0 mg/day |
23.8 |
-3.87 |
16 HOW SUPPLIED
AZILECT 1 mg Tablets:
White to off-white, round, flat, beveled tablets, debossed with "GIL 1" on
one side and plain on the other side.
Supplied as bottles of 30 tablets (NDC 54868-6206-0).
Storage:
Store at 25°C (77°F) with excursions permitted to 15°-30°C (59°-86°F).
17 INFORMATION FOR PATIENTS
Enter section text here
17.1 Coadministration of Antidepressants and Other Drugs
Patients should inform their physician if they are taking, or planning to take, any prescription or over-the-counter drugs, especially antidepressants and over-the-counter cold medications, since there is a potential for interaction with AZILECT. Because patients should not use meperidine or certain other analgesics with AZILECT, they should contact their healthcare provider before taking analgesics [see Warnings and Precautions (5.1)].
17.2 Ciprofloxacin or Other CYP1A2 Inhibitors
Patients should be informed that they should contact their healthcare provider of AZILECT if they take ciprofloxacin or a similar drug that could increase blood levels of rasagiline because of the need to adjust the dose of AZILECT [see Warnings and Precautions (5.2)].
17.3 Risk of Hypertensive Crisis and Nonselective Monoamine Oxidase Inhibition Above the Recommended Doses
Patients should be advised not to exceed the maximum recommended daily dose of 1 mg/day (0.5 mg/day for subjects with mild hepatic impairment and subjects using concomitant ciprofloxacin and other CYP1A2 inhibitors).
The risk of using higher than recommended daily doses of AZILECT should be explained, and a brief description of the hypertensive/cheese reaction provided.
The possibility exists that very tyramine-rich foods (e.g., aged cheese such as Stilton) could possibly cause an increase in blood pressure. Patients should be advised to avoid certain foods (e.g., aged cheese) containing a very large amount of tyramine while taking recommended doses of AZILECT because of the potential for large increases in blood pressure. If patients eat foods very rich in tyramine and do not feel well soon after eating, they should contact their healthcare provider [see Warnings and Precautions (5.4)].
17.4 Melanoma
It is not known if melanoma is associated with Parkinson's disease or the medicines used to treat Parkinson's disease. Patients being treated with AZILECT should be advised to have periodic skin examinations. [see Warnings and Precautions (5.5)].
17.5 Dyskinesia
Patients taking AZILECT as adjunct to levodopa should be advised that there is a possibility of dyskinesia or increased dyskinesia [see Warnings and Precautions (5.6)].
17.6 Lowering of Blood Pressure and Postural/Orthostatic Hypotension
Patients should be advised that they may develop postural (orthostatic) hypotension with or without symptoms such as dizziness, nausea, syncope, and sometimes sweating. Hypotension and/or orthostatic symptoms may occur more frequently during initial therapy or with an increase in dose at any time (cases have been seen after weeks of treatment). Accordingly, patients should be cautioned against standing up rapidly after sitting or lying down, especially if they have been doing so for prolonged periods, and especially, at the initiation of treatment with AZILECT [see Warnings and Precautions (5.7)].
17.7 Elevation of Blood Pressure
Patients should be alerted to the possibility of increases in blood pressure during treatment with AZILECT. Exacerbation of hypertension may occur. Medication dose adjustment may be necessary if elevation of blood pressure is sustained over multiple evaluations [see Warnings and Precautions (5.8)].
17.8 Hallucinations / Psychotic-Like Behavior
Patients should be informed that hallucinations or other manifestations of psychotic-like behavior can occur when taking AZILECT. Patients should also be advised that, if they have a major psychotic disorder, that AZILECT should not ordinarily be used because of the risk of exacerbating the psychosis. Patients with a major psychotic disorder should also be aware that many treatments for psychosis may decrease the effectiveness of AZILECT [see Warnings and Precautions (5.9)].
17.9 Withdrawal-Emergent Hyperpyrexia and Confusion
Patients should be told to contact their healthcare provider if they wish to discontinue Azilect.
17.10 Missing Dose
Patients should be instructed to take AZILECT as prescribed. If a dose is missed, the patient should not double-up the dose of AZILECT. The next dose should be taken at the usual time on the following day.
17.11 Impulse Control / Compulsive Behaviors
There have been reports of patients experiencing intense urges to gamble, increased sexual urges, other intense urges, and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone and that are generally used for the treatment of Parkinson's disease (including AZILECT). Although it is not proven that the medications caused these events, these urges were reported to have stopped in some cases when the dose was reduced or the medication was stopped. Prescribers should ask patients about the development of new or increased gambling urges, sexual urges, or other urges while being treated with rasagiline. Patients should inform their physician if they experience new or increased gambling urges, increased sexual urges, or other intense urges while taking rasagiline. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking rasagiline.
U.S. Patent Nos. 5387612, 5453446, 5457133, 5532415, 5786390, 6126968
Marketed by: TEVA Neuroscience, Inc., Kansas City, MO 64131
Distributed
by: TEVA Pharmaceuticals USA, Inc., North Wales, PA 19454
Product of
Israel
Relabeling of "Additional Barcode Label" by:
Physicians Total Care, Inc.
Tulsa, OK 74146
PRINCIPAL DISPLAY PANEL
AZILECT
®
(rasagiline
tablets)
1 mg
30 Tablets Rx only

Azilectrasagiline mesylate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||