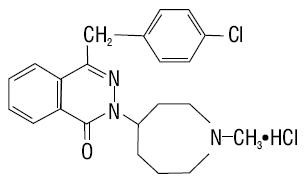
Azelastine Hydrochloride
Azelastine Hydrochloride Nasal Solution (Nasal Spray), 0.1% (137 mcg per spray)For Intranasal Use Only
FULL PRESCRIBING INFORMATION: CONTENTS*
- AZELASTINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- AZELASTINE HYDROCHLORIDE INDICATIONS AND USAGE
- AZELASTINE HYDROCHLORIDE CONTRAINDICATIONS
- PRECAUTIONS
- AZELASTINE HYDROCHLORIDE ADVERSE REACTIONS
- AZELASTINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- AZELASTINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PATIENT: How To Use Instructions
- PRINCIPAL DISPLAY PANEL-Showbox
FULL PRESCRIBING INFORMATION
AZELASTINE HYDROCHLORIDE DESCRIPTION
22243
CLINICAL PHARMACOLOGY
1in vitro1
Pharmacokinetics and Metabolism
max
The major active metabolite, desmethylazelastine, was not measurable (below assay limits) after single-dose intranasal administration of azelastine hydrochloride. After intranasal dosing of azelastine hydrochloride to steady-state, plasma concentrations of desmethylazelastine range from 20 to 50% of azelastine concentrations. When azelastine hydrochloride is administered orally, desmethylazelastine has an elimination half-life of 54 hours. Limited data indicate that the metabolite profile is similar when azelastine hydrochloride is administered via the intranasal or oral route.
In vitro studies with human plasma indicate that the plasma protein binding of azelastine and desmethylazelastine are approximately 88% and 97%, respectively.
Azelastine hydrochloride administered intranasally at doses above two sprays per nostril twice daily for 29 days resulted in greater than proportional increases in Cmax and area under the curve (AUC) for azelastine.
Studies in healthy subjects administered oral doses of azelastine hydrochloride demonstrated linear responses in Cmax and AUC.
Special Populations
Following oral administration, pharmacokinetic parameters were not influenced by age, gender, or hepatic impairment.
Based on oral, single-dose studies, renal insufficiency (creatinine clearance <50 mL/min) resulted in a 70 to 75% higher Cmax and AUC compared to normal subjects. Time to maximum concentration was unchanged.
Pharmacodynamics
In a placebo-controlled study (95 subjects with allergic rhinitis), there was no evidence of an effect of azelastine hydrochloride nasal solution (2 sprays per nostril twice daily for 56 days) on cardiac repolarization as represented by the corrected QT interval (QTc) of the electrocardiogram. At higher oral exposures (≥4 mg twice daily), a nonclinically significant mean change on the QTc (3 to 7 millisecond increase) was observed.
Interaction studies investigating the cardiac repolarization effects of concomitantly administered oral azelastine hydrochloride and erythromycin or ketoconazole were conducted. Oral erythromycin had no effect on azelastine pharmacokinetics or QTc based on analysis of serial electrocardiograms. Ketoconazole interfered with the measurement of azelastine plasma levels; however, no effects on QTc were observed (see PRECAUTIONS, Drug Interactions).
Clinical Trials
Seasonal Allergic Rhinitis
Trials Supporting Two Sprays Per Nostril Twice Daily
U.S. placebo-controlled clinical trials of azelastine hydrochloride nasal solution included 322 patients with seasonal allergic rhinitis who received two sprays per nostril twice a day for up to 4 weeks. These trials included 55 pediatric patients ages 12 to 16 years. Azelastine hydrochloride nasal solution showed significant improvement compared to placebo in the two primary efficacy variables- the Total Symptom Complex (TSC) and the Major Symptom Complex (MSC).The results for the MSC are shown in Table 1 as the mean change from Baseline in the average of individual symptoms of nose blows, sneezes, runny nose/sniffles, itchy nose and watery eyes as assessed by patients on a 0 to 5 categorical scale.
| Azelastine HCl Nasal Solution(Nasal Spray) | Placebo Nasal Spray | Outcomes | ||
|---|---|---|---|---|
| Azelastine vs. Placebo | ||||
| Mean (SD) | Difference between Treatments |
P value | ||
|
Study 26: 12 Hour AM and PM Reflective MSC
|
||||
| Sample Size |
N=63 |
N=60 |
||
| Baseline |
11.48 (4.13) |
10.84 (4.53) |
||
| Change from Baseline |
-3.05 (3.51) |
-1.07 (3.52) |
1.98 |
0.0024 |
|
Study 31: 12 Hour AM and PM Reflective MSC
|
||||
| Sample Size |
N=63 |
N=63 |
||
| Baseline |
12.5 (4.5) |
12.18 (4.64) |
||
| Change from Baseline |
-4.1 (3.46) |
-2.07 (4.01) |
2.03 |
0.0023 |
|
Study 33: 12 Hour AM and PM Reflective MSC
|
||||
| Sample Size |
N=66 |
N=66 |
||
| Baseline |
12.04 (4.03) |
11.66 (3.96) |
||
| Change from Baseline |
-3.31 (3.74) |
-1.96 (3.57) |
1.35 |
0.0374 |
Trials Supporting One Spray Per Nostril Twice Daily
| Azelastine HCl Nasal Solution (Nasal Spray) | Placebo Nasal Spray | Outcomes | ||
|---|---|---|---|---|
| Azelastine vs. Placebo | ||||
| LS Mean (SD) | Difference between Treatments |
P value | ||
|
Study 419: 12 Hour AM and PM Reflective TNSS
|
||||
| Sample Size |
N=138 |
N=141 |
||
| Baseline |
16.34 (4.222) |
17.21 (4.316) |
||
| Change from Baseline |
-2.69 (4.789) |
-1.31 (4.285) |
1.38 |
0.0017 |
|
Study 420: 12 Hour AM and PM Reflective TNSS
|
||||
| Sample Size |
N=137 |
N=136 |
||
| Baseline |
16.62 (4.197) |
16.84 (4.768) |
||
| Change from Baseline |
-3.68 (4.163) |
-2.5 (4.011) |
1.18 |
0.0173 |
Other Supporting Studies
Vasomotor Rhinitis
AZELASTINE HYDROCHLORIDE INDICATIONS AND USAGE
AZELASTINE HYDROCHLORIDE CONTRAINDICATIONS
PRECAUTIONS
Activities Requiring Mental Alertness
Information for Patients
Patients should be instructed to use azelastine hydrochloride nasal solution only as prescribed. For the proper use of the nasal spray and to attain maximum improvement, the patient should read and follow carefully the accompanying patient instructions. Patients should be instructed to prime the delivery system before initial use and after storage for 3 or more days (see PATIENT INSTRUCTIONS FOR USE). Patients should also be instructed to store the bottle upright at room temperature with the pump tightly closed and out of the reach of children. In case of accidental ingestion by a young child, seek professional assistance or contact a poison control center immediately.
Drug InteractionsDrug Interactions
Concurrent use of azelastine hydrochloride nasal solution with alcohol or other CNS depressants should be avoided because additional reductions in alertness and additional impairment of CNS performance may occur.
Cimetidine (400 mg twice daily) increased the mean Cmax and AUC of orally administered azelastine hydrochloride (4 mg twice daily) by approximately 65%. Ranitidine hydrochloride (150 mg twice daily) had no effect on azelastine pharmacokinetics.
Interaction studies investigating the cardiac effects, as measured by the corrected QT interval (QTc), of concomitantly administered oral azelastine hydrochloride and erythromycin or ketoconazole were conducted. Oral erythromycin (500 mg three times daily for seven days) had no effect on azelastine pharmacokinetics or QTc based on analyses of serial electrocardiograms. Ketoconazole (200 mg twice daily for seven days) interfered with the measurement of azelastine plasma concentrations; however, no effects on QTc were observed.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In 2 year carcinogenicity studies in rats and mice azelastine hydrochloride did not show evidence of carcinogenicity at oral doses up to 30 mg/kg and 25 mg/kg, respectively (approximately 240 and 100 times the maximum recommended daily intranasal dose in adults and children on a mg/m2 basis).
Azelastine hydrochloride showed no genotoxic effects in the Ames test, DNA repair test, mouse lymphoma forward mutation assay, mouse micronucleus test, or chromosomal aberration test in rat bone marrow.
22Pregnancy Category C
Azelastine hydrochloride has been shown to cause developmental toxicity. Treatment of mice with an oral dose of 68.6 mg/kg (approximately 280 times the maximum recommended daily intranasal dose in adults on a mg/m2 basis) caused embryo-fetal death, malformations (cleft palate; short or absent tail; fused, absent or branched ribs), delayed ossification and decreased fetal weight. This dose also caused maternal toxicity as evidenced by decreased body weight. Neither fetal nor maternal effects occurred at a dose of 3 mg/kg (approximately 10 times the maximum recommended daily intranasal dose in adults on a mg/m2 basis).
In rats, an oral dose of 30 mg/kg (approximately 240 times the maximum recommended daily intranasal dose in adults on a mg/m2 basis) caused malformations (oligo-and brachydactylia), delayed ossification and skeletal variations, in the absence of maternal toxicity. At 68.6 mg/kg (approximately 560 times the maximum recommended daily intranasal dose in adults on a mg/m2 basis) azelastine hydrochloride also caused embryo-fetal death and decreased fetal weight; however, the 68.6 mg/kg dose caused severe maternal toxicity. Neither fetal nor maternal effects occurred at a dose of 3 mg/kg (approximately 25 times the maximum recommended daily intranasal dose in adults on a mg/m2 basis).
In rabbits, oral doses of 30 mg/kg and greater (approximately 500 times the maximum recommended daily intranasal dose in adults on a mg/m2 basis) caused abortion, delayed ossification and decreased fetal weight; however, these doses also resulted in severe maternal toxicity. Neither fetal nor maternal effects occurred at a dose of 0.3 mg/kg (approximately 5 times the maximum recommended daily intranasal dose in adults on a mg/m2 basis).
Nursing Mothers
Pediatric Use
Geriatric Use
AZELASTINE HYDROCHLORIDE ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact CARACO Pharmaceutical Laboratories Ltd. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Seasonal Allergic Rhinitis
Azelastine Hydrochloride Nasal Solution Two Sprays Per Nostril Twice Daily
| ADVERSE EVENT |
Azelastine Hydrochloride Nasal Spray n = 391 |
Vehicle Placebo n = 353 |
| Bitter Taste |
19.7 |
0.6 |
| Headache |
14.8 |
12.7 |
| Somnolence |
11.5 |
5.4 |
| Nasal Burning |
4.1 |
1.7 |
| Pharyngitis |
3.8 |
2.8 |
| Dry Mouth |
2.8 |
1.7 |
| Paroxysmal Sneezing |
3.1 |
1.1 |
| Nausea |
2.8 |
1.1 |
| Rhinitis |
2.3 |
1.4 |
| Fatigue |
2.3 |
1.4 |
| Dizziness |
2 |
1.4 |
| Epistaxis |
2 |
1.4 |
| Weight Increase |
2 |
0 |
Azelastine Hydrochloride Nasal Solution One Spray Per Nostril Twice Daily
Adverse experience information for azelastine hydrochloride nasal solution at a dose of one spray per nostril twice daily is derived from two placebo-controlled 2-week clinical studies which included 276 patients. None of the patients receiving azelastine hydrochloride nasal solution were discontinued from these studies due to adverse reactions. Three patients receiving vehicle placebo were discontinued due to adverse reactions. Bitter taste was reported in 8.3% of patients compared to none in the placebo group. Somnolence was reported in 0.4% of patients compared to none in the placebo group.
A total of 176 patients 5 to 12 years of age were exposed to azelastine hydrochloride nasal solution at a dose of 1 spray each nostril twice daily in 3 placebo-controlled studies. In these studies, adverse events that occurred more frequently in patients treated with azelastine hydrochloride nasal solution than with placebo, and that were not represented in the adult adverse event table above include rhinitis/cold symptoms (17% vs 9.5%), cough (11.4% vs 8.3%), conjunctivitis (5.1% vs 1.8%), and asthma (4.5% vs 4.1%).
The following events were observed infrequently (<2% and exceeding placebo incidence) in patients who received azelastine hydrochloride nasal solution dosed at 1 or 2 sprays per nostril twice daily in U.S. clinical trials.
Cardiovascular: flushing, hypertension, tachycardia.
Dermatological: contact dermatitis, eczema, hair and follicle infection, furunculosis, skin laceration.
Digestive: constipation, gastroenteritis, glossitis, ulcerative stomatitis, vomiting, increased SGPT, aphthous stomatitis, diarrhea, toothache.
Metabolic and Nutritional: increased appetite.
Musculoskeletal: myalgia, temporomandibular dislocation, rheumatoid arthritis.
Neurological: hyperkinesia, hypoesthesia, vertigo.
Psychological: anxiety, depersonalization, depression, nervousness, sleep disorder, thinking abnormal.
Respiratory: bronchospasm, coughing, throat burning, laryngitis, bronchitis, dry throat, nocturnal dyspnea, nasopharyngitis, nasal congestion, pharyngolaryngeal pain, sinusitis, nasal dryness, paranasal sinus hypersecretion, post nasal drip.
Special Senses: conjunctivitis, eye abnormality, eye pain, watery eyes, taste loss.
Urogenital: albuminuria, amenorrhea, breast pain, hematuria, increased urinary frequency.
AZELASTINE HYDROCHLORIDE ADVERSE REACTIONS
Vasomotor Rhinitis
Adverse experience information for azelastine hydrochloride nasal solution is derived from two placebo-controlled clinical studies which included 216 patients who received azelastine hydrochloride nasal solution at a dose of 2 sprays per nostril twice daily for up to 28 days. The incidence of discontinuation due to adverse reactions in patients receiving azelastine hydrochloride nasal solution was not different from vehicle placebo (2.8% vs 2.9%, respectively).
| ADVERSE EVENT |
Azelastine Hydrochloride Nasal Spray n = 216 |
Vehicle Placebo n = 210 |
| Bitter Taste |
19.4 |
2.4 |
| Headache |
7.9 |
7.6 |
| Dysesthesia |
7.9 |
3.3 |
| Rhinitis |
5.6 |
2.4 |
| Epistaxis |
3.2 |
2.4 |
| Sinusitis |
3.2 |
1.9 |
| Somnolence |
3.2 |
1 |
Events observed infrequently (<2% and exceeding placebo incidence) in patients who received azelastine hydrochloride nasal solution (2 sprays/nostril twice daily) in U.S. clinical trials in vasomotor rhinitis were similar to those observed in U.S. clinical trials in seasonal allergic rhinitis.
In controlled trials involving nasal and oral azelastine hydrochloride formulations, there were infrequent occurrences of hepatic transaminase elevations. The clinical relevance of these reports has not been established.
OVERDOSAGE
22
AZELASTINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Seasonal Allergic Rhinitis
Vasomotor Rhinitis
The recommended dose of azelastine hydrochloride nasal solution in adults and children 12 years and older with vasomotor rhinitis is two sprays per nostril twice daily.
Before initial use, the delivery system should be primed with 4 sprays or until a fine mist appears. When 3 or more days have elapsed since the last use, the pump should be reprimed with 2 sprays or until a fine mist appears.
CAUTION: Avoid spraying in the eyes.
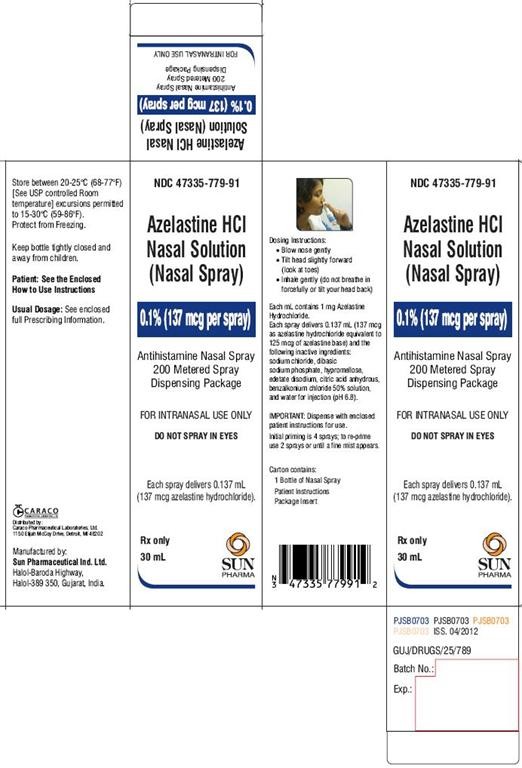
HOW SUPPLIED
Azelastine Hydrochloride Nasal Solution (Nasal Spray), 0.1% (137 mcg per spray), (NDC 47335-779-91) is supplied as a package containing 200 metered sprays in a high-density polyethylene (HDPE) bottle fitted with a metered-dose spray pump unit. A leaflet of patient instructions is also provided. The spray pump unit consists of a nasal spray pump fitted with a white safety clip and a white plastic dust cover.
The Azelastine Hydrochloride Nasal Solution (Nasal Spray), 0.1% (137 mcg per spray), bottle contains 30 mg (1 mg/mL) of azelastine hydrochloride. The bottle can deliver 200 metered sprays. Each spray delivers a mean of 0.137 mL solution containing 137 mcg of azelastine hydrochloride.
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Ind. Ltd.
PATIENT: How To Use Instructions
FOR INTRANASAL USE ONLY
IMPORTANT: FOLLOW INSTRUCTIONS CAREFULLY TO ENSURE PROPER DOSING.
DOSING: The dosage of azelastine hydrochloride nasal solution is 1 spray per nostril twice daily for pediatric patients (ages 5 to 11 years) with seasonal allergic rhinitis. For patients age 12 and older with seasonal allergic rhinitis the dosage is one or two sprays per nostril twice daily. For patients age 12 and older with nonallergic vasomotor rhinitis the dosage is two sprays per nostril twice daily. Keep your head tilted downward when spraying. Alternate sprays between nostrils. Breathe gently to avoid drawing any medication into the throat.
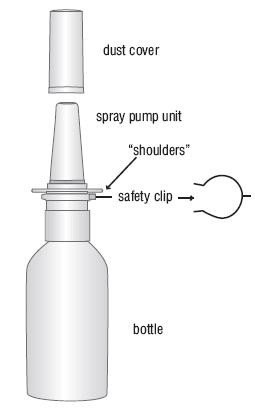
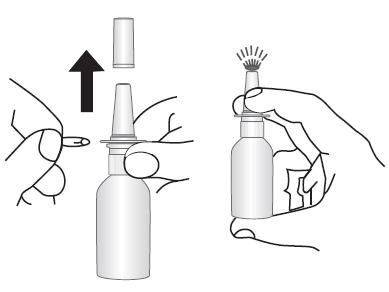
TO PRIME:
- Remove and retain the blue spray tip dust cover and blue safety clip.
- Prime for initial use by putting two fingers on the shoulders of the spray pump unit and place your thumb on the bottom of the bottle. Press upward with thumb, release, and repeat until a fine mist appears (4 sprays or less). Now your pump is primed and ready to use.
- If the solution is delivered in a stream of liquid, it may fail to provide maximum benefit and cause some discomfort. A fine mist can be produced only by a rapid and firm pumping action.
- When 3 or more days have elapsed since the last use, the pump should be reprimed with 2 sprays or until a fine mist appears.
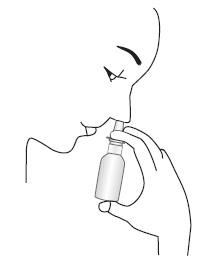
TO USE:
- Gently blow nose to clear nostrils.
- Keep your head tilted downward toward your toes.
- Place the spray tip ¼ to ½ inch into one nostril. Hold bottle vertically upright (as shown), allowing spray tip to aim toward the back of the nose. Close other nostril with finger, rapidly press once with thumb and sniff gently at the same time. You may feel a brief burning or stinging sensation after using the unit.
- Repeat in other nostril.
- For patients aged 12 and over who were instructed by their doctor to administer 2 sprays in each nostril, repeat Steps 2 and 3 for second spray, again alternating sprays between nostrils.
- Breathe in gently, and do not tilt head back after dosing to avoid drawing medication into the throat (where it will be tasted).
CAUTION: Keep bottle tightly closed and away from children. In case of accidental ingestion by a young child, seek professional assistance or contact a poison control center immediately. Do not spray in eyes.
NOTE: Keep the dust cover and safety clip on the spray pump unit when not in use. After each use and before replacing the dust cover, wipe the spray tip with a clean tissue or cloth.
For more information, call 1-800-818-4555.
TO CLEAN:
- If spray nozzle becomes clogged, DO NOT ATTEMPT TO CLEAR IT USING A POINTED OBJECT. Remove the spray pump unit from the bottle.
- Soak only the spray pump unit in warm water. Squirt several times while holding under water.
- Make sure the spray pump unit is dry.
- Reinsert the pump into the open bottle and tighten by turning clockwise.
- To avoid leakage, firm pressure is required to ensure that the pump is fully threaded onto the bottle.
- Follow instructions for priming.

Keep bottle tightly closed and away from children.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Ind. Ltd.
PRINCIPAL DISPLAY PANEL-Showbox
NDC 47335-779-91
Azelastine HCl Nasal Solution (Nasal Spray)
0.1% (137 mcg per spray)
DO NOT SPRAY IN EYES
Rx only
30 mL
SUN PHARMA
Azelastine HydrochlorideAzelastine Hydrochloride SPRAY, METERED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
