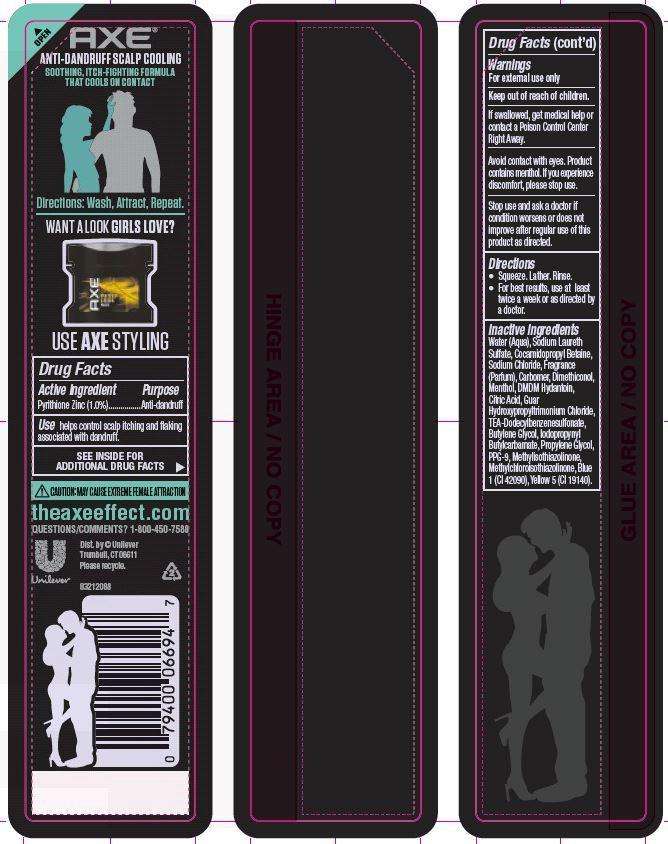
Axe
Axe Freeze Anti-Dandruff shampoo+conditioner
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient
Pyrithione Zinc (1.0%)
Purpose
Purpose
Anti-dandruff
Uses
Use helps control scalp itching and flaking associated with dandruff.
Warnings
For external use only
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center Right Away.
Avoid contact with eyes. Product contains menthol. If you experience discomfort, please stop use.
Stop use and ask a doctor if condition worsens or does not improve after regular use of this product as directed.
Direction
· Squeeze. Lather. Rinse
· For best results, use at least twice a week or as directed by a doctor.
Inactive ingredients
Water (Aqua), Sodium Laureth Sulfate, Cocamidopropyl Betaine, Sodium Chloride, Fragrance (Parfum), Carbomer, Dimethiconol, Menthol, DMDM Hydantoin, Citric Acid, Guar Hydroxypropyltrimonium Chloride, TEA-Dodecylbenzenesulfonate, Butylene Glycol, Iodopropynyl Butylcarbamate, Propylene Glycol, PPG-9, Methylisothiazolinone, Methylchloroisothiazolinone, Blue 1 (CI 42090), Yellow 5 (CI 19140).
theaxeeffect.com
QUESTIONS/COMMENTS?
1-800-450-7580
12 FL OZ PDP

12 FL OZ back
AxePyrithione Zinc SHAMPOO
| |||||||||||||||||||||||||||||||||||||||||||||||||