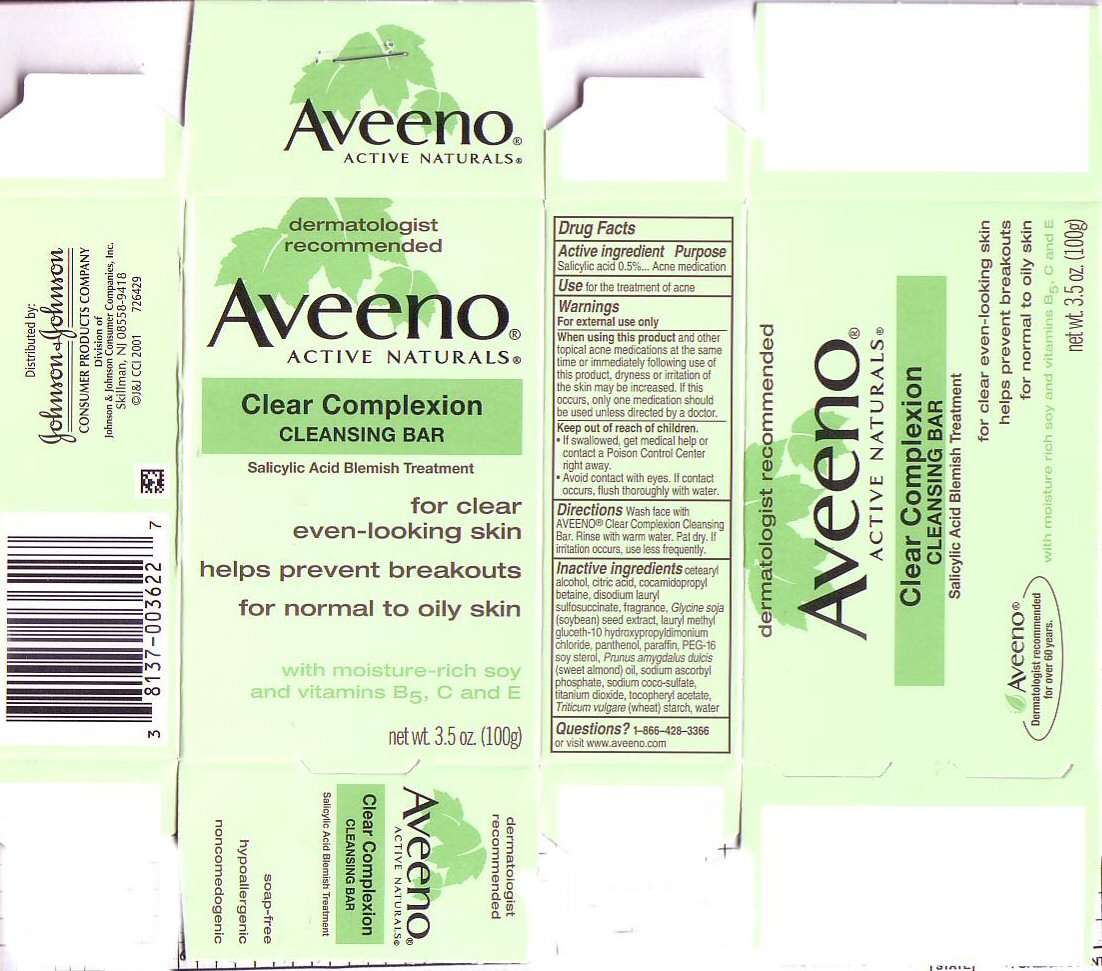
AVEENO
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT
SALICYLIC ACID 0.5%
PURPOSE
ACNE MEDICATION
USE
FOR THE TREATMENT OF ACNE
WARNINGS
FOR EXTERNAL USE ONLYKEEP OUT OF REACH OF CHILDREN
- IF SWALLOWED, GET HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
- AVOID CONTACT WITH EYES. IF CONTACT OCCURS, FLUSH THOROUGHLY WITH WATER.
DIRECTIONS
WASH FACE WITH AVEENO CLEAR COMPLEXION CLEANSING BAR. RINSE WITH WATER. PAT DRY. IF IRRITATION OCCURS, USE LESS FREQUENTLY.
INACTIVE INGREDIENTS
CETEARYL ALCOHOL, CITRIC ACID, COCAMIDOPROPYL BETAINE, DISODIUM LAURYL SULFOSUCCINATE, FRAGRANCE, GLYCINE SOJA (SOYBEAN) SEED EXTRACT, LAURYL METHYL GLUCETH-10 HYDROXYPROPYLDIMONIUM CHLORIDE, PANTHENOL, PARAFFIN, PEG-16 SOY STEROL, PRUNUS AMYGDALUS DULCIS (SWEET ALMOND) OIL, SODIUM ASCORBYL PHOSPHATE, SODIUM COCO-SULFATE, TITANIUM DIOXIDE, TOCOPHERYL ACETATE, TRICICUM VULGARE (WHEAT) STARCH, WATER
QUESTIONS?
1-866-428-3366 OR VISIT www.aveeno.com
AVEENOAVEENO SOAP
| |||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!