AVAR
AVAR™ Cleanser (sodium sulfacetamide 10% and sulfur 5%)
FULL PRESCRIBING INFORMATION: CONTENTS*
- Rx Only
- CLINICAL PHARMACOLOGY:
- INDICATIONS:
- AVAR CONTRAINDICATIONS:
- WARNINGS:
- PRECAUTIONS:
- AVAR ADVERSE REACTIONS:
- AVAR DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
FULL PRESCRIBING INFORMATION
Rx Only
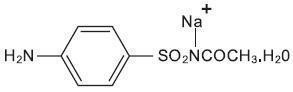
DESCRIPTION:
89232
CLINICAL PHARMACOLOGY:
Propionibacterium acnes
INDICATIONS:
CONTRAINDICATIONS:
WARNINGS:
KEEP OUT OF REACH OF CHILDREN.
PRECAUTIONS:
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.General:
Carcinogenesis, Mutagenesis and Impairment of Fertility:
Pregnancy:
Nursing Mothers:
Pediatric Use:
ADVERSE REACTIONS:
To report SUSPECTED ADVERSE REACTIONS, contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DOSAGE AND ADMINISTRATION:
HOW SUPPLIED:

AVARSULFACETAMIDE SODIUM, SULFUR EMULSION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!