Atenolol
Atenolol Tablets, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- ATENOLOL DESCRIPTION
- CLINICAL PHARMACOLOGY
- ATENOLOL INDICATIONS AND USAGE
- ATENOLOL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ATENOLOL ADVERSE REACTIONS
- OVERDOSAGE
- ATENOLOL DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 25 mg (30 Tablet Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 50 mg (30 Tablet Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 100 mg (30 Tablet Bottle)
FULL PRESCRIBING INFORMATION
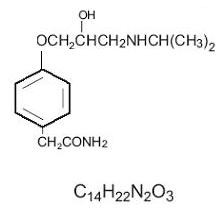
ATENOLOL DESCRIPTION
1
CLINICAL PHARMACOLOGY
12
Pharmacokinetics and Metabolism
2 DOSAGE AND ADMINISTRATION
Pharmacodynamics
1 21
Atenolol Geriatric Pharmacology
ATENOLOL INDICATIONS AND USAGE
Hypertension
Angina Pectoris Due to Coronary Atherosclerosis
Acute Myocardial Infarction
DOSAGE AND ADMINISTRATION, CONTRAINDICATIONS WARNINGS
ATENOLOL CONTRAINDICATIONS
WARNINGS
WARNINGS
Cardiac Failure
In Patients Without a History of Cardiac Failure
DOSAGE AND ADMNISTRATION.
|
Cessation of Therapy with Atenolol Patients with coronary artery disease, who are being treated with atenolol, should be advised against abrupt discontinuation of therapy. Severe exacerbation of angina and the occurrence of myocardial infarction and ventricular arrhythmias have been reported in angina patients following the abrupt discontinuation of therapy with beta-blockers. The last two complications may occur with or without preceding exacerbation of the angina pectoris. As with other beta-blockers, when discontinuation of atenolol is planned, the patients should be carefully observed and advised to limit physical activity to a minimum. If the angina worsens or acute coronary insufficiency develops, it is recommended that atenolol be promptly reinstituted, at least temporarily. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue atenolol therapy abruptly even in patients treated only for hypertension. (See DOSAGE AND ADMINISTRATION .) |
PRECAUTIONS
Bronchospastic Diseases
PATIENTS WITH BRONCHOSPASTIC DISEASE SHOULD, IN GENERAL, NOT RECEIVE BETA-BLOCKERS. Because of its relative beta 1 selectivity, however, atenolol may be used with caution in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment. Since beta 1 selectivity is not absolute, the lowest possible dose of atenolol should be used with therapy initiated at 50 mg and a beta 2-stimulating agent (bronchodilator) should be made available. If dosage must be increased, dividing the dose should be considered in order to achieve lower peak blood levels.
Major Surgery
Diabetes and Hypoglycemia
Thyrotoxicosis
DOSAGE AND ADMINISTRATION
Untreated Pheochromocytoma
Pregnancy and Fetal Injury
PRECAUTIONS, Nursing Mothers
PRECAUTIONS
General
Impaired Renal Function
DOSAGE AND ADMINISTRATION
Drug Interactions
WARNINGS
Carcinogenesis, Mutagenesis, Impairment of Fertility
in vivo S typhimurium
Animal Toxicology
Usage in Pregnancy
WARNINGS - Pregnancy and Fetal Injury
Nursing Mothers
WARNINGS, Pregnancy and Fetal Injury
Pediatric Use
Geriatric Use
CLINICAL PHARMACOLOGY INDICATIONS AND USAGE
ATENOLOL ADVERSE REACTIONS
| |
Volunteered (U.S. Studies) |
Total – Volunteered and Elicited (Foreign+U.S . Studies) |
||
| Atenolol (n=164) % |
Placebo (n=206) % |
Atenolol (n=399) % |
Placebo (n=407) % |
|
| CARDIOVASCULAR |
|
|
|
|
| Bradycardia |
3 |
0 |
3 |
0 |
| Cold Extremities |
0 |
0.5 |
12 |
5 |
| Postural Hypotension |
2 |
1 |
4 |
5 |
| Leg Pain |
0 |
0.5 |
3 |
1 |
| CENTRAL NERVOUS SYSTEM/NEUROMUSCULAR |
|
|
|
|
| Dizziness |
4 |
1 |
13 |
6 |
| Vertigo |
2 |
0.5 |
2 |
0.2 |
| Lightheadedness |
1 |
0 |
3 |
0.7 |
| Tiredness |
0.6 |
0.5 |
26 |
13 |
| Fatigue |
3 |
1 |
6 |
5 |
| Lethargy |
1 |
0 |
3 |
0.7 |
| Drowsiness |
0.6 |
0 |
2 |
0.5 |
| Depression |
0.6 |
0.5 |
12 |
9 |
| Dreaming |
0 |
0 |
3 |
1 |
| GASTROINTESTINAL |
|
|
|
|
| Diarrhea |
2 |
0 |
3 |
2 |
| Nausea |
4 |
1 |
3 |
1 |
| RESPIRATORY (see
WARNINGS
) |
|
|
|
|
| Wheeziness |
0 |
0 |
3 |
3 |
| Dyspnea |
0.6 |
1 |
6 |
4 |
Acute Myocardial Infarction
| |
Conventional Therapy Plus Atenolol (n=244) |
Conventional Therapy Alone (n=233) |
||
| Bradycardia |
43 |
(18%) |
24 |
(10%) |
| Hypotension |
60 |
(25%) |
34 |
(15%) |
| Bronchospasm |
3 |
(1.2%) |
2 |
(0.9%) |
| Heart Failure |
46 |
(19%) |
56 |
(24%) |
| Heart Block |
11 |
(4.5%) |
10 |
(4.3%) |
| BBB + Major Axis Deviation |
16 |
(6.6%) |
28 |
(12%) |
| Supraventricular Tachycardia |
28 |
(11.5%) |
45 |
(19%) |
| Atrial Fibrillation |
12 |
(5%) |
29 |
(11%) |
| Atrial Flutter |
4 |
(1.6%) |
7 |
(3%) |
| Ventricular Tachycardia |
39 |
(16%) |
52 |
(22%) |
| Cardiac Reinfarction |
0 |
(0%) |
6 |
(2.6%) |
| Total Cardiac Arrests |
4 |
(1.6%) |
16 |
(6.9%) |
| Nonfatal Cardiac Arrests |
4 |
(1.6%) |
12 |
(5.1%) |
| Deaths |
7 |
(2.9%) |
16 |
(6.9%) |
| Cardiogenic Shock |
1 |
(0.4%) |
4 |
(1.7%) |
| Development of Ventricular Septal Defect |
0 |
(0%) |
2 |
(0.9%) |
| Development of Mitral Regurgitation |
0 |
(0%) |
2 |
(0.9%) |
| Renal Failure |
1 |
(0.4%) |
0 |
(0%) |
| Pulmonary Emboli |
3 |
(1.2%) |
0 |
(0%) |
| Reasons for Reduced Dosage | ||||
|---|---|---|---|---|
| IV Atenolol Reduced Dose (< 5 mg)* |
Oral Partial Dose | |||
| Hypotension/Bradycardia |
105 |
(1.3%) |
1168 |
(14.5%) |
| Cardiogenic Shock |
4 |
(.04%) |
35 |
(.44%) |
| Reinfarction |
0 |
(0%) |
5 |
(.06%) |
| Cardiac Arrest |
5 |
(.06%) |
28 |
(.34%) |
| Heart Block (> first degree) |
5 |
(.06%) |
143 |
(1.7%) |
| Cardiac Failure |
1 |
(.01%) |
233 |
(2.9%) |
| Arrhythmias |
3 |
(.04%) |
22 |
(.27%) |
| Bronchospasm |
1 |
(.01%) |
50 |
(.62%) |
POTENTIAL ADVERSE EFFECTS
Hematologic:
Allergic:
Central Nervous System:
Gastrointestinal:
Other:
Miscellaneous: DOSAGE AND ADMINISTRATION
OVERDOSAGE
2
ATENOLOL DOSAGE AND ADMINISTRATION
Hypertension
Angina Pectoris
Acute Myocardial Infarction
Elderly Patients or Patients with Renal Impairment
2
| Creatinine Clearance (mL/min/1.73 m2) |
Atenolol Elimination Half-Life (h) |
Maximum Dosage |
| 15-35 |
16-27 |
50 mg daily |
| <15 |
>27 |
25 mg daily |
Cessation of Therapy in Patients with Angina Pectoris
HOW SUPPLIED
Atenolol Tablets USP, 25 mg
Atenolol Tablets USP, 50 mg
Atenolol Tablets USP, 100 mg
Store at
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 25 mg (30 Tablet Bottle)
NDC 51138-096-30

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 50 mg (30 Tablet Bottle)
NDC 51138-097-30

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 100 mg (30 Tablet Bottle)
NDC 51138-098-30

AtenololAtenolol TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AtenololAtenolol TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AtenololAtenolol TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!