Atenolol
FULL PRESCRIBING INFORMATION: CONTENTS*
- CESSATION OF THERAPY WITH ATENOLOL
- ATENOLOL DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- ATENOLOL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ATENOLOL ADVERSE REACTIONS
- POTENTIAL ADVERSE EFFECTS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ATENOLOL DESCRIPTION
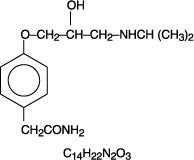
CLINICAL PHARMACOLOGY
Pharmacokinetics and Metabolism
DOSAGE AND ADMINISTRATION).
Pharmacodynamics
Atenolol Geriatric Pharmacology
INDICATIONS & USAGE
HypertensionAngina Pectoris Due to Coronary Atherosclerosis
Acute Myocardial Infarction
DOSAGE AND ADMINISTRATION,CONTRAINDICATIONS, andWARNINGS). In general, there is no basis for treating patients like those who were excluded from the ISIS-1 trial (blood pressure less than 100 mm Hg systolic, heart rate less than 50 bpm) or have other reasons to avoid beta blockade. As noted above, some subgroups (e.g., elderly patients with systolic blood pressure below 120 mm Hg) seemed less likely to benefit.
ATENOLOL CONTRAINDICATIONS
WARNINGS).WARNINGS
Cardiac FailureIn Patients Without a History of Cardiac Failure
DOSAGE AND ADMINISTRATION).
CESSATION OF THERAPY WITH ATENOLOL
DOSAGE AND ADMINISTRATION).
Concomitant Use of Calcium Channel Blockers
PRECAUTIONS).
Bronchospastic Diseases
PATIENTS WITH BRONCHOSPASTIC DISEASE SHOULD, IN GENERAL, NOT RECEIVE BETA BLOCKERS. Because of its relative beta1 selectivity, however, atenolol may be used with caution in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment. Since beta1 selectivity is not absolute, the lowest possible dose of atenolol should be used with therapy initiated at 50 mg and a beta2-stimulating agent (bronchodilator) should be made available. If dosage must be increased, dividing the dose should be considered in order to achieve lower peak blood levels.
Major Surgery
Diabetes and Hypoglycemia
Thyrotoxicosis
DOSAGE AND ADMINISTRATION).
Untreated Pheochromocytoma
Pregnancy and Fetal Injury
PRECAUTIONS,Nursing Mothers.)
1Although similar effects were not seen in rabbits, the compound was not evaluated in rabbits at doses above 25 mg/kg/day or 12.5 times the maximum recommended human antihypertensive dose.2
1
2
PRECAUTIONS
GeneralImpaired Renal Function
DOSAGE AND ADMINISTRATION).
Drug Interactions
WARNINGS).
Carcinogenesis, Mutagenesis, Impairment of Fertility
3did not indicate a carcinogenic potential of atenolol. A third (24 month) rat study, employing doses of 500 and 1,500 mg/kg/day (250 and 750 times the maximum recommended human antihypertensive dose4) resulted in increased incidences of benign adrenal medullary tumors in males and females, mammary fibroadenomas in females, and anterior pituitary adenomas and thyroid parafollicular cell carcinomas in males. No evidence of a mutagenic potential of atenolol was uncovered in the dominant lethal test (mouse), in vivo cytogenetics test (Chinese hamster) or Ames test (S typhimurium).
5) was unaffected by atenolol administration.
3
4
5
Animal Toxicology
6) and increased incidence of atrial degeneration of hearts of male rats at 300 but not 150 mg atenolol/kg/day (150 and 75 times the maximum recommended human antihypertensive dose,7respectively).
6
7
Usage in Pregnancy
WARNINGS:Pregnancy and Fetal Injury.
Nursing Mothers
WARNINGS,Pregnancy and Fetal Injury).
Pediatric Use
Geriatric Use
CLINICAL PHARMACOLOGY), 33% (2,644) were 65 years of age and older. It was not possible to identify significant differences in efficacy and safety between older and younger patients; however, elderly patients with systolic blood pressure < 120 mmHg seemed less likely to benefit (seeINDICATIONS AND USAGE).
ATENOLOL ADVERSE REACTIONS
Volunteered
(U.S. Studies)Total-Volunteered
and Elicited
(Foreign + U.S. Studies)Atenolol
(n = 164)
% Placebo
(n = 206)
% Atenolol
(n = 399)
% Placebo
(n = 407)
% WARNINGS)
Acute Myocardial Infarction
Conventional
Therapy Plus
Atenolol
(n = 244)Conventional
Therapy
Alone
(n = 233)
*
Reasons for Reduced Dosage IV Atenolol
Reduced Dose
(< 5 mg) * Oral
Partial Dose
POTENTIAL ADVERSE EFFECTS
Hematologic
Allergic
Central Nervous System
Gastrointestinal
Other
Miscellaneous
DOSAGE AND ADMINISTRATION).
OVERDOSAGE
Bradycardia
Heart Block (Second or Third Degree)
Cardiac Failure
Hypotension
Bronchospasm
Hypoglycemia
DOSAGE & ADMINISTRATION
HypertensionAngina Pectoris
Acute Myocardial Infarction
Elderly Patients or Patients with Renal Impairment
Creatinine Clearance
(mL/min/1.73m2) Atenolol
Elimination
Half-Life (h) Maximum Dosage
Cessation of Therapy in Patients with Angina Pectoris
HOW SUPPLIED
25 mg:round, white, unscored tablets debossed GG L7 on one side and plain on the reverse side, and supplied as:
50 mg:round, white, scored tablets debossed GG 263 on one side and plain on the reverse side, and supplied as:
100 mg:round, white, unscored tablets debossed GG 264 on one side and plain on the reverse side, and supplied as:
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

AtenololATENOLOL TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!