Atenolol
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- ATENOLOL DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- PHARMACODYNAMICS
- INDICATIONS & USAGE
- ATENOLOL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- ATENOLOL ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ATENOLOL DESCRIPTION
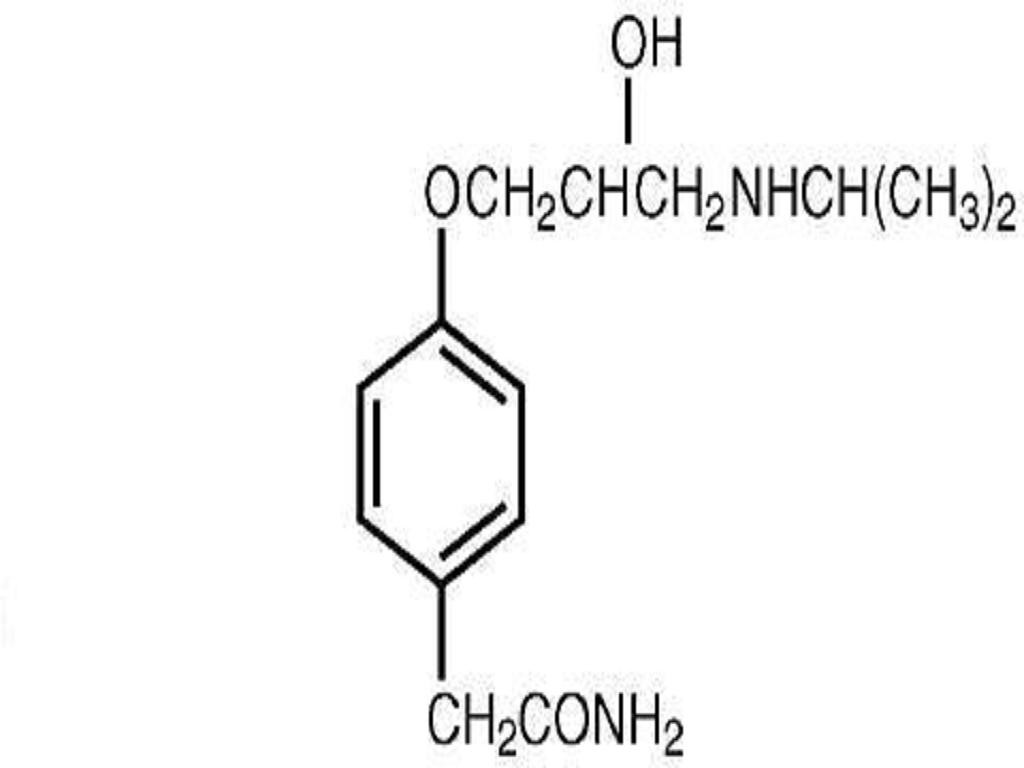
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
Pharmacokinetics and MetabolismDOSAGE AND ADMINISTRATION
PHARMACODYNAMICS
INDICATIONS & USAGE
HypertensionAngina Pectoris Due to Coronary Atherosclerosis
Acute Myocardial Infarction
DOSAGE AND ADMINISTRATIONCONTRAINDICATIONSWARNINGS
ATENOLOL CONTRAINDICATIONS
WARNINGSWARNINGS
Cardiac FailureIn Patients Without a History of Cardiac Failure
DOSAGE AND ADMNISTRATION
DOSAGE AND ADMINISTRATION
Concomitant Use of Calcium Channel Blockers
PRECAUTIONS
Bronchospastic Diseases
Anesthesia and Major Surgery
OVERDOSAGE
Diabetes and Hypoglycemia
Thyrotoxicosis
DOSAGE AND ADMINISTRATION
Untreated Pheochromocytoma
Pregnancy and Fetal Injury
PRECAUTIONS, Nursing Mothers
PRECAUTIONS
GeneralImpaired Renal Function
DOSAGE AND ADMINISTRATION
DRUG INTERACTIONS
WARNINGS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
PREGNANCY
WARNINGS - Pregnancy and Fetal Injury
NURSING MOTHERS
WARNINGS, Pregnancy and Fetal Injury
PEDIATRIC USE
GERIATRIC USE
CLINICAL PHARMACOLOGYINDICATIONS AND USAGE).
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Evaluation of patients with hypertension or myocardial infarction should always include assessment of renal function.
ATENOLOL ADVERSE REACTIONS
WARNINGS
Acute Myocardial Infarction
POTENTIAL ADVERSE EFFECTS
INDICATIONS AND USAGE
OVERDOSAGE
DOSAGE & ADMINISTRATION
HypertensionAngina Pectoris
Acute Myocardial Infarction
Elderly Patients or Patients with Renal Impairment
Cessation of Therapy in Patients with Angina Pectoris
HOW SUPPLIED
Atenolol Tablets, USPPACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

AtenololAtenolol TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!