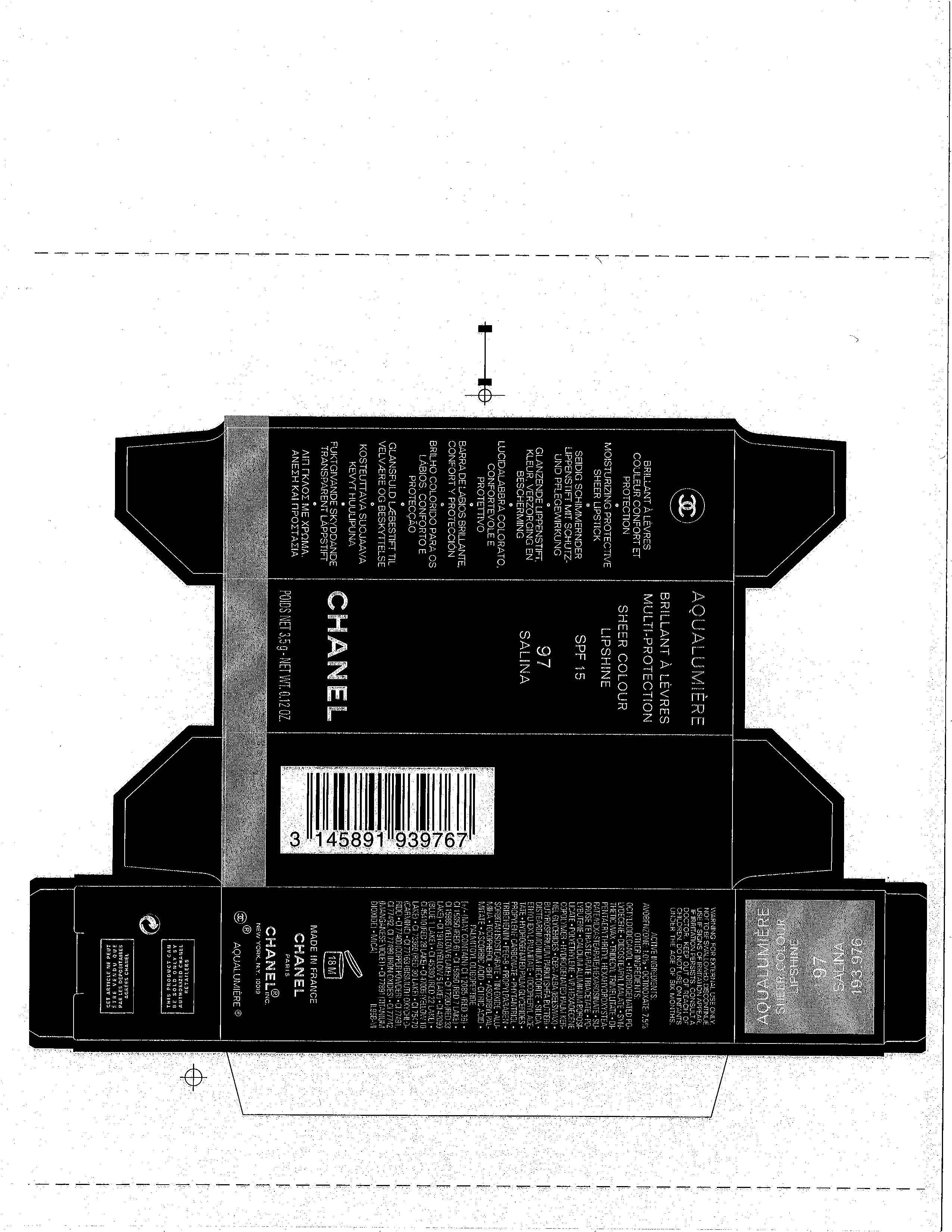
AQUALUMIERE
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT(S)
AVOBENZONE 1%
OCTINOXATE 7.5%
WARNINGS
FOR EXTERNAL USE ONLY. NOT TO BE SWALLOWED. DISCONTINUE USE IF SIGNS OF IRRITATION APPEAR. IF IRRITATION PERSISTS, CONSULT A DOCTOR. KEEP OUT OF REACH OF CHILDREN. DO NOT USE ON INFANTS UNDER THE AGE OF SIX MONTHS.
DIRECTIONS FOR USE
APPLY EVENLY TO LIPS.
INACTIVE INGREDIENTS
OCTYLDODECANOL, HYDROGENATED POLYDECENE, DIISOSTEARYL MALATE, SYNTHETIC WAX, TRIDECYL TRIMELLITATE, DIPENTAERYTHRITYL HEXAHYDROXYSTEARATE/HEXASTEARATE/HEXAROSINATE, SUCROSE TETRASTEARATE TRIACETATE, POLYBUTENE, CALCIUM ALUMINUM BOROSILICATE, POLYETHYLENE, VP/HEXADECEN COPOLYMER, HYDROGENATED PALM KERNEL GLYCERIDES, CERA ALBA (BEESWAX), BUTYROSPERMUM PARKII (SHEA BUTTER), DISTEARDIMONIUM HECTORITE, SILICA, ETHYLHEXYL PALMITATE, TOCOPHERYL ACETATE, HYDROGENATED PALM GLYCERIDES, PROPYLENE CARBONATE, PHYTANTRIOL, TRIBEHENIN, PEG-8, PROPYLPARABEN, SORBITAN ISOSTEARATE, TIN OXIDE, ALUMINA, TOCOPHEROL, BHT, ASCORBYL PALMITATE, ASCORBIC ACID, CITRIC ACID, PALMITOYL OLIGOPEPTIDE [+/- (MAY CONTAIN) CI 12085 (RED 36), CI 15850 (RED 6), CI 15850 (RED 7 LAKE), CI 15985 (YELLOW 6 LAKE), CI 17200 (RED 33 LAKE), CI 19140 (YELLOW 5 LAKE), CI 42090 (BLUE 1 LAKE), CI 45380 (RED 22 LAKE), CI 45410 (RED 28 LAKE) CI 14005 (YELLOW 10 LAKE), CI 73360 (RED 30 LAKE), CI 75470 (CARMINE), CI 77163 (BISMUTH OXYCHLORIDE), CI 77400 (COPPER POWDER), CI 77491, CI 77492, CI 77499 (IRON OXIDES), CI 77742 (MANGANESE VIOLET), CI 77891 (TITANIUM DIOXIDE), MICA]
PRINCIPAL DISPLAY PANEL
AQUALUMIERE
SHEER COLOUR
LIPSHINE
SPF 15
(SHADE)
CHANEL
POIDS NET 3,5g NET WT. 0.12 OZ.
AQUALUMIERESHEER COLOUR LIPSHINE LIPSTICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||