Apres Peel Soothing Balm
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients: Hydrocortisone (0.5%)
Purpose
Purpose: Antipruritic (anti-itch)
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Uses
Uses: For temporary relief of itching/discomfort associated with minor skin irritation, inflammation and rashes due to: insect bites, soaps, detergents, seborrheic dematitis, eczema, etc. Other uses of this product should only be under the advice and supervision of a physician.
Warnings: For external use only.
Stop use of this product or any other hydrocortisone product and consult a physician if condition worses, or if symptoms persist for more than 7 days or clear up and occur again within a few days.
Do not use for the treatment of diaper rash. Instead, consult a physician.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions: Adults and children 2 years of age or older: Apply to the affected are no more than 3 to 4 times daily. Children under 2 years of age: Do not use, consult a physician.
Inactive Ingredients: Aloe Barbadensis Leaf Juice Powder, Olea Europaea (Olive) Fruit Oil, Water/Aqua/Eau, Propylene Glycol, Glyceryl Stearate, Ricinus Communis (Castor) Seed Oil, Triticum Vulgare (Wheat) Germ Oil, PEG-100 Stearate, Stearic Acid, Isopropyl Palmitate, Cetyl Alochol, Glycine Soja (Soybean) Oil, Hydrolyzed Milk Protein, Prunus Armeniaca (Apricot) Kernel Oil, Citrus Medica Limonum (Lemon) Peel Oil, Triethanolamine, Methylparaben, Propylparaben, Imidazolidinyl Urea
Principal Display Panel 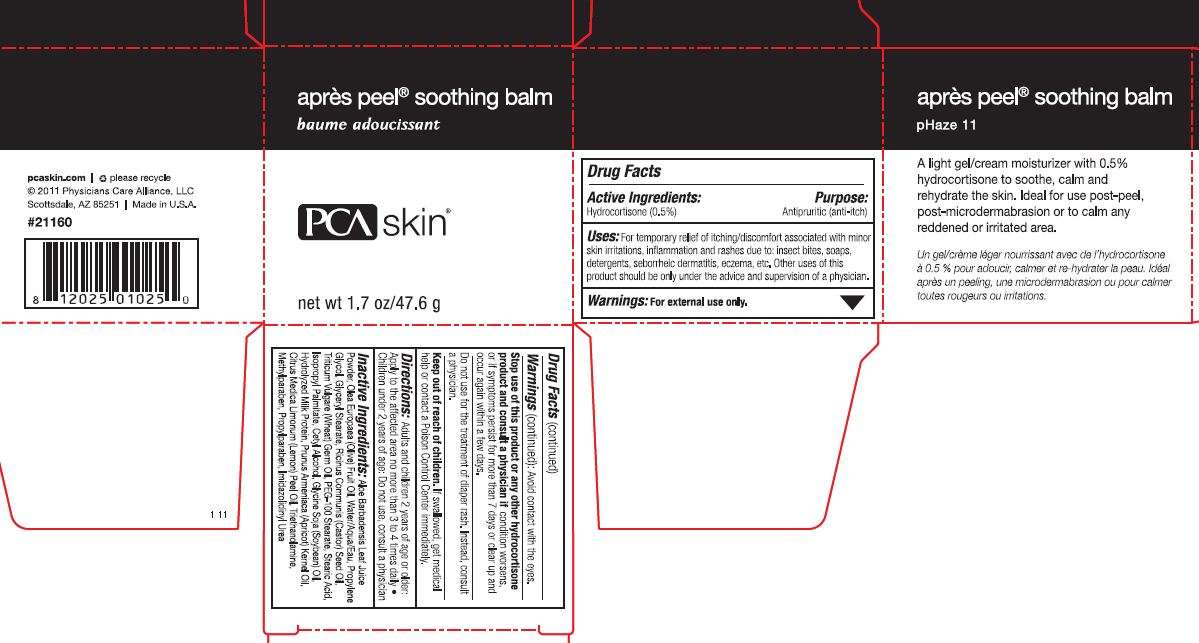
Apres Peel Soothing BalmHydrocortisone CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||