Antipyrine and Benzocaine
ANTIPYRINE AND BENZOCAINE OTIC SOLUTION
FULL PRESCRIBING INFORMATION: CONTENTS*
- ANTIPYRINE AND BENZOCAINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- ANTIPYRINE AND BENZOCAINE INDICATIONS AND USAGE
- ANTIPYRINE AND BENZOCAINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ANTIPYRINE AND BENZOCAINE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 15 mL Bottle Carton
FULL PRESCRIBING INFORMATION
EAR SOLUTION
Topical Decongestant and Analgesic
Rx Only
ANTIPYRINE AND BENZOCAINE DESCRIPTION
Antipyrine and Benzocaine Otic Solution is an otic solution containing Antipyrine, Benzocaine, Oxyquinoline Sulfate, and Anhydrous Glycerin for use in the ear. The solution congeals at 0°C (32°F), but returns to normal consistency, unchanged, at room temperature.
Antipyrine is an analgesic with local anesthetic action, it is chemically 2,3-dimethyl-1-phenyl-3-pyrazolin-5-one. The active ingredient is represented by the structural formula:
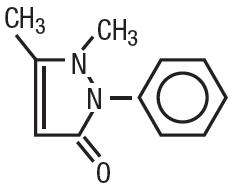
|
| C11H12N2O MW=188.22 |
Antipyrine occurs as colorless crystals or white powder, has a slightly bitter taste and is soluble in water and alcohol.
Benzocaine is a local anesthetic. It is chemically ethyl p-aminobenzoate or Benzoic acid, 4-amino-, ethyl ester. The active ingredient is represented by the structural formula:
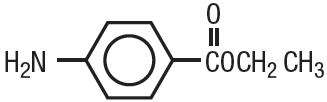
|
| C9H11NO2 MW=165.19 |
It occurs as white crystals or white crystalline powder and is slightly soluble in water and soluble in organic solvents.
EACH mL CONTAINS:
Actives: Antipyrine 54 mg, Benzocaine 14 mg; Inactives: Glycerine (anhydrous), Oxyquinoline Sulfate.
CLINICAL PHARMACOLOGY
Antipyrine and Benzocaine Otic Solution combines the hygroscopic property of anhydrous glycerin with the analgesic action of antipyrine and benzocaine to relieve pressure, reduce inflammation and congestion, and to alleviate pain and discomfort in acute otitis media.
Antipyrine and Benzocaine Otic Solution does not blanch the tympanic membrane or mask the landmarks and, therefore, does not distort the otoscopic picture.
ANTIPYRINE AND BENZOCAINE INDICATIONS AND USAGE
Acute Otitis media of various etiologies
-
- prompt relief of pain and reduction of inflammation in the congestive and serous stages. -
- adjuvant therapy during systemic antibiotic administration for resolution of the infection.
Because of the close anatomical relationship of the eustachian tube to the nasal cavity, otitis media is a frequent problem, especially in children in whom the tube is shorter, wider, and more horizontal than in adults.
Removal of Cerumen
-
- facilitates the removal of excessive or impacted cerumen.
ANTIPYRINE AND BENZOCAINE CONTRAINDICATIONS
The product is contraindicated in any person with hypersensitivity to any of the components or substances related to them. This product is contraindicated in the presence of spontaneous perforation of the tympanic membrane or discharge.
WARNINGS
FOR USE IN EARS ONLY-NOT FOR USE IN EYES
Discontinue promptly if sensitization or irritation occurs.
PRECAUTIONS
Information for Patients
Avoid contaminating the dropper with material from the ear, fingers or other source.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long term studies have been conducted.
Pregnancy
Category C
Animal reproduction studies have not been conducted with Antipyrine and Benzocaine Otic Solution. It is also not known whether Antipyrine and Benzocaine Otic Solution can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Antipyrine and Benzocaine Otic Solution should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Antipyrine and Benzocaine Otic Solution is administered to a nursing woman.
ANTIPYRINE AND BENZOCAINE DOSAGE AND ADMINISTRATION
Acute otitis media
Instill Antipyrine and Benzocaine Otic Solution, permitting the solution to run along the wall of the ear canal until it is filled. Avoid touching the ear with dropper. Then moisten a cotton pledget with Antipyrine and Benzocaine Otic Solution and insert into meatus. Repeat every one to two hours until pain and congestion are relieved.
Removal of Cerumen
Before
Instill Antipyrine and Benzocaine Otic Solution three times daily for two or three days to help detach cerumen from wall of ear canal and facilitate removal.
After
Antipyrine and Benzocaine Otic Solution is useful for drying out the ear canal or relieving discomfort.
Before and after removal of cerumen, a cotton pledget moistened with Antipyrine and Benzocaine Otic Solution should be inserted into the meatus following instillation.
HOW SUPPLIED
Antipyrine & Benzocaine Otic Solution is supplied in plastic bottles containing 15 mL (NDC# 66424-520-35).
A tamper evident seal is on the bottle cap. Do not dispense if the seal is broken.
Storage
Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature]. Protect from heat and light. Protect from freezing.
Manufactured for:
SDA Laboratories
Greenwich, CT 06830
Ph: 800-687-0176
Iss. 06/10
PRINCIPAL DISPLAY PANEL - 15 mL Bottle Carton
SDA
LABORATORIES
NDC 66424-520-35
Antipyrine &
Benzocaine
Otic Solution, USP
EACH mL CONTAINS
Actives:
Antipyrine USP 54 mg
Benzocaine USP 14 mg
Inactive ingredients: Glycerine
(anhydrous), Oxyquinoline Sulfate.
FOR USE IN THE EARS ONLY
Tamper evident seal on bottle cap.
Do not use if seal is broken.
Rx only
15 mL (1/2 fl. oz.)

Antipyrine and BenzocaineAntipyrine and Benzocaine SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||