Antimicrobial
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Antimicrobial Uses
- Warnings
- Directions
- Inactive ingredients
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Triclosan 0.3%
Purpose
Antimicrobial
Antimicrobial Uses
- Handwash to help reduce bacteria that potentially can cause disease.
Warnings
- For external use only.
When using this product
- Avoid contact with eyes. In case of eye contact, flush with water.
- Consult your doctor before use if you have any deep wounds, animal bites or serious burns.
Stop use and ask a doctor if
- irritation or redness develops. If condition persists, seek medical attention.
Keep out of reach of children.
- If swallowed, seek immediate medical attention or call a Poison Control Center.
Directions
- Wet hands, apply soap, rub hands together vigorously for at least 15 seconds. Rinse and dry hands thoroughly with a disposable paper towel.
Inactive ingredients
Water/Aqua, Sodium Lauryl Sulfate, Sodium C14-16 Sulfonate, Cocamidopropyl Betaine, Cocamide DEA, Glycerin, Disodium R-MEA Sulfosuccinate, Ammonium Chloride, Fragrance, Methylchloroisothiazolinone and Methylisothazolinone, Citric Acid, FD&C Yellow #5, FD&C Red #4.
Package/Label Principal Display Panel
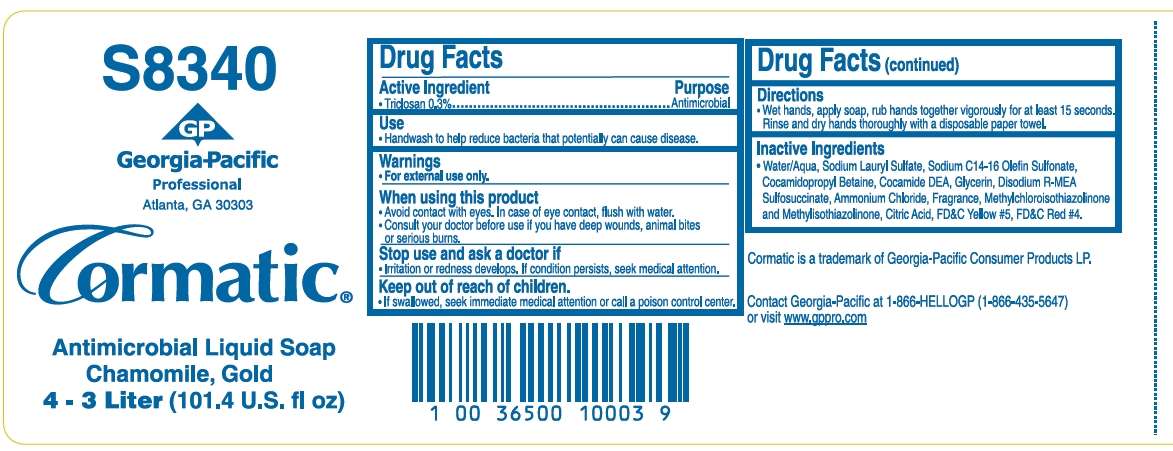
Antimicrobial Liquid Soap
S8340
GP
Georgia-Pacific
Professional
Atlanta, GA 30303
Cormatic®
Antimicrobial Liquid Soap
Chamomile, Gold
4 - 3 Liter (101.4 U.S. fl oz)
AntimicrobialTriclosan SOAP
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!