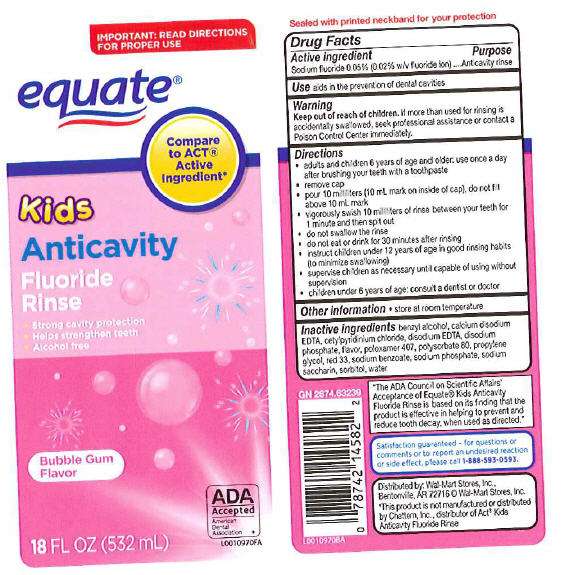
Anticavity Fluoride Rinse
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredient Purpose
Sodium Fluoride 0.05% (0.2% w/v fluoride ion) Anticavity rinse
Uses
Use
Warning
Keep out of reach of children. If
more than used for rinsing is accidentally swallowed, seek professional
assistance or contact a Poison Control Center immediately
- adults and children 6 year of age and older: use once a day after brushing your teeth with a toothpaste
- remove cap
- pour 10 milliliters (10 mL mark on inside of cap), do not fill above 10 mL mark
- vigorously swish 10 milliliters of rinse between your teeth for 1 minute and then spit out
- do not swallow the rinse
- do not eat or drink for 30 minutes after rinsing
- instruct children under 12 years of age in good rinsing habits (to minimize swallowing)
- supervise children as necessary until capable of using without supervision
- children under 6 year of age: consult a dentist or doctor
Other information store at room temperature
Inactive ingredients benzuyl alcohol, calcium disodium EDTA, cetylpyridinium chloride, disodium EDTA, disodium phosphate, flavor, poloxomer 407, polysorbate 80, propylene glycol, red33, sodium benzoate, sodijm phosphate, sodium saccharin, sorbitol, water
IMPORTANT: READ DIRECTIONS FOR PROPER USE
KIDS
ANTICAVITY
FLUORIDE RINSE
•Strong cavity protection
•Helps strengthen teeth
•Alcohol free
Bubble Gum Flavor
18 FL OZ (532 mL)
Anticavity Fluoride RinseAnticavity Fluoride Rinse MOUTHWASH
| |||||||||||||||||||||||||||||||||||||||||||||||||