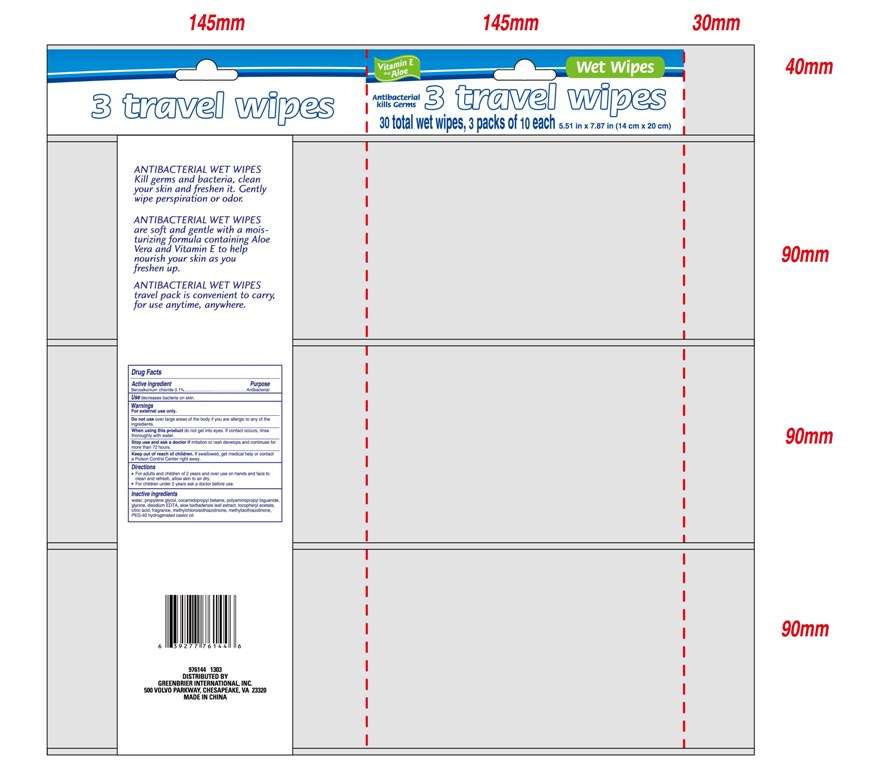
Antibacterial Wet Wipes
ZHEJIANG GREENFACE HOUSEWARES CO., LTD.
ANTIBACTERIAL WET WIPES
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active ingredient
Benzalkonium chloride 0.1%
Purpose
Antibacterial
Use
Use decreases bacteria on skin.
Warnings
For external use only.
Do not use over large areas of the body if you are allergic to any of the ingredients.
When using this product do not get into eyes. If contact occurs, rinse thoroughly with water.
Stop use and ask a doctor if irritation or rash develops and continues for more than 72 hours.
Keep out of reach of children. If swallowed, get medical help or contact a PoisonControlCenterright away.
Keep out of reach of children
Keep out of reach of children. If swallowed, get
medical help or contact aPoisonControlCenter
right away.
Directions
For adults and children of 2 years and over use on hands and face to clean and refresh, allow skin to air dry.
For children under 2 years ask a doctor before use.
water, propylene glycol, cocamidopropyl betaine, polyaminopropyl biguanide, glycine, disodium EDTA, aloe barbadensis leaf extract, tocopheryl acetate, citric acid, fragrance, methylchloroisothiazolinone, methylisothiazolinone, PEG-40 hydrogenated castor oil.



Antibacterial Wet WipesBENZALKONIUM CHLORIDE SWAB
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||