Amoxicillin and Clavulanate Potassium
FULL PRESCRIBING INFORMATION: CONTENTS*
- AMOXICILLIN AND CLAVULANATE POTASSIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- AMOXICILLIN AND CLAVULANATE POTASSIUM INDICATIONS AND USAGE
- AMOXICILLIN AND CLAVULANATE POTASSIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- AMOXICILLIN AND CLAVULANATE POTASSIUM ADVERSE REACTIONS
- OVERDOSAGE
- AMOXICILLIN AND CLAVULANATE POTASSIUM DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- AMOXICILLIN AND CLAVULANATE POTASSIUM DESCRIPTION OF CLINICAL STUDIES
- REFERENCES
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 600 mg/42.9 mg per 5 mL (200 mL Bottle)
FULL PRESCRIBING INFORMATION
AMOXICILLIN AND CLAVULANATE POTASSIUM DESCRIPTION
SRRRp
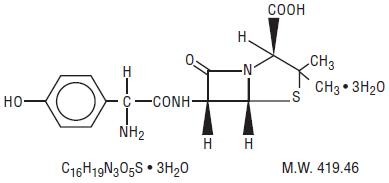
Streptomyces clavuligerus-ZRR
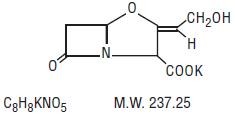
Inactive Ingredients:
PRECAUTIONS, Information for the Patient, Phenylketonurics
CLINICAL PHARMACOLOGY
| Parameter* | Amoxicillin | Clavulanate |
|---|---|---|
|
*Arithmetic mean ± standard deviation, except Tmax values which are medians (ranges). |
||
| Cmax (mcg/mL) |
15.7 ± 7.7 |
1.7 ± 0.9 |
| Tmax (hr) |
2 (1 to 4) |
1.1 (1 to 4) |
| AUC0-t (mcg•hr/mL) |
59.8 ± 20 |
4 ± 1.9 |
| T½ (hr) |
1.4 ± 0.3 |
1.1 ± 0.3 |
| CL/F (L/hr/kg) |
0.9 ± 0.4 |
1.1 ± 1.1 |
| Timepoint |
Amoxicillin concentration in plasma (mcg/mL) |
Amoxicillin concentration in MEF (mcg/mL) |
|
| 1 hour |
mean median range |
7.7 9.3 1.5 to 14 (n = 5) |
3.2 3.5 0.2 to 5.5 (n = 4) |
| 2 hour |
mean median range |
15.7 13 11 to 25 (n = 7) |
3.3 2.4 1.9 to 6 (n = 5) |
| 3 hour |
mean median range |
13 12 5.5 to 21 (n = 5) |
5.8 6.5 3.9 to 7.4 (n = 5) |
Microbiology
in vitro INDICATIONS AND USAGE
Aerobic Gram-Positive Microorganisms
Streptococcus pneumoniae
Aerobic Gram-Negative Microorganisms
Haemophilus influenzae
Moraxella catarrhalis
in vitro but their clinical significance is unknown.
in vitro
Aerobic Gram-Positive Microorganisms
Staphylococcus aureus
NOTE:
Streptococcus pyogenes
NOTE: S. pyogenesS. pyogenes
Susceptibility Test Methods
in vitro
Dilution Technique
1,2 S. pneumoniaeH. influenzae
Table 3
Diffusion Technique
2,3Table 3
| Pathogen | Minimum Inhibitory Concentration (mcg/mL) | Disk Diffusion (Zone Diameter in mm) |
||||
|---|---|---|---|---|---|---|
| S | I | R | S | I | R | |
|
Streptococcus pneumoniae (non-meningitis isolates) |
≤ 2/1 |
4/2 |
≥ 8/4 |
Not applicable (NA) |
||
|
Haemophilus influenzae
|
≤ 4/2 |
NA |
≥ 8/4 |
≥ 20 |
NA |
≤ 19 |
NOTE:S. pneumoniaeS. pneumoniae
NOTE: H. influenzae
1-3Table 4.Table 4
| *ATCC is a trademark of the American Type Culture Collection. † When using Haemophilus Test Medium (HTM). |
||
|
Quality Control Organism
|
Minimum
Inhibitory Concentration Range
(mcg/mL)
|
Disk Diffusion (Zone Diameter Range in mm)
|
|
Escherichia coli ATCC®* 35218†
(H. influenzae quality control) |
4/2 to 16/8 |
17 to 22 |
|
Haemophilus influenzae ATCC 49247 |
2/1 to 16/8 |
15 to 23 |
|
Streptococcus pneumoniae ATCC 49619 |
0.03/0.016 to 0.12/0.06 |
NA |
AMOXICILLIN AND CLAVULANATE POTASSIUM INDICATIONS AND USAGE
S. pneumoniaeH. influenzae M. catarrhalis
- antibiotic exposure for acute otitis media within the preceding 3 months, and either of the following:
- age ≤ 2 years
- daycare attendance
CLINICAL PHARMACOLOGY, Microbiology
NOTE: S. pneumoniae S. pneumoniae
S. pneumoniae
AMOXICILLIN AND CLAVULANATE POTASSIUM CONTRAINDICATIONS
WARNINGS
SERIOUS ANAPHYLACTIC REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS, AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Clostridium difficileC. difficile
C. difficile C. difficile
C. difficileC. difficile
CONTRAINDICATIONS ADVERSE REACTIONS, Liver
PRECAUTIONS
General
PseudomonasCandida
Information for the Patient
Phenylketonurics
Drug Interactions
Drug/Laboratory Test Interactions
®®
.
Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitroin vivoin vitro
Teratogenic Effects
Pregnancy (Category B)
Labor and Delivery
Nursing Mothers
Pediatric Use
DESCRIPTION OF CLINICAL STUDIES
CLINICAL PHARMACOLOGY
AMOXICILLIN AND CLAVULANATE POTASSIUM ADVERSE REACTIONS
Gastrointestinal
WARNINGS
Hypersensitivity Reactions
WARNINGS
Liver
CONTRAINDICATIONS
Renal
OVERDOSAGE
Hemic and Lymphatic Systems
Central Nervous System
Miscellaneous
OVERDOSAGE
4
AMOXICILLIN AND CLAVULANATE POTASSIUM DOSAGE AND ADMINISTRATION
Amoxicillin and clavulanate potassium for oral suspension, 600 mg/42.9 mg per 5 mL, does not contain the same amount of clavulanic acid (as the potassium salt) as any of the other amoxicillin and clavulanate potassium suspensions. Amoxicillin and clavulanate potassium for oral suspension, 600 mg/42.9 mg per 5 mL contains 42.9 mg of clavulanic acid per 5 mL, whereas the amoxicillin and clavulanate potassium, 200 mg/28.5 mg per 5 mL suspension contains 28.5 mg of clavulanic acid per 5 mL and the 400 mg/57 mg per 5 mL suspension contains 57 mg of clavulanic acid per 5 mL. Therefore, the amoxicillin and clavulanate potassium 200 mg/28.5 mg per 5 mL and 400 mg/57 mg per 5 mL suspensions should not be substituted for amoxicillin and clavulanate potassium for oral suspension, 600 mg/42.9 mg per 5 mL, as they are not interchangeable.
Dosage
Pediatric patients 3 months and older
| Body Weight (kg) |
Volume of Amoxicillin and Clavulanate Potassium for Oral Suspension, 600 mg/42.9 mg per 5 mL providing 90 mg/kg/day |
|---|---|
| 8 |
3 mL twice daily |
| 12 |
4.5 mL twice daily |
| 16 |
6 mL twice daily |
| 20 |
7.5 mL twice daily |
| 24 |
9 mL twice daily |
| 28 |
10.5 mL twice daily |
| 32 |
12 mL twice daily |
| 36 |
13.5 mL twice daily |
Pediatric patients weighing 40 kg and more
Adults
WARNINGS
Directions for Mixing Oral Suspension
| Bottle Size |
Amount of Water Required for Reconstitution |
| 75 mL |
71 mL |
| 125 mL |
112 mL |
| 200 mL |
176 mL |
NOTE:
Administration
HOW SUPPLIED
Amoxicillin and Clavulanate Potassium for Oral Suspension USP, 600 mg/42.9 mg per 5 mL
STORAGE
DESCRIPTION OF CLINICAL STUDIES
S. pneumoniaeS. pneumoniaeTable 5
| Bacteriologic Eradication on Therapy | |||
|---|---|---|---|
| Pathogen | n/N | % | 95% CI* |
| * CI = confidence intervals; 95% CIs are not adjusted for multiple comparisons. |
|||
| All S. pneumoniae
|
121/123 |
98.4 |
(94.3, 99.8) |
|
S. pneumoniae with penicillin MIC = 2 mcg/mL |
19/19 |
100 |
(82.4, 100) |
|
S. pneumoniae with penicillin MIC = 4 mcg/mL |
12/14 |
85.7 |
(57.2, 98.2) |
|
H. influenzae
|
75/81 |
92.6 |
(84.6, 97.2) |
|
M. catarrhalis
|
11/11 |
100 |
(71.5, 100) |
| 2 to 4 Days Post-Therapy (Primary Endpoint) |
|||
|---|---|---|---|
| Pathogen | n/N | % | 95% CI† |
| * S. pneumoniae strains with penicillin MICs of 2 or 4 mcg/mL are considered resistant to penicillin. † CI = confidence intervals; 95% CIs are not adjusted for multiple comparisons. ‡ Clinical assessments at 15 to 18 days post-therapy may have been confounded by viral infections and new episodes of acute otitis media with time elapsed post-treatment. |
|||
| All S. pneumoniae
|
122/137 |
89.1 |
(82.6, 93.7) |
|
S. pneumoniae with penicillin MIC = 2 mcg/mL |
17/20 |
85 |
(62.1, 96.8) |
|
S. pneumoniae with penicillin MIC = 4 mcg/mL |
11/14 |
78.6 |
(49.2, 95.3) |
|
H. influenzae
|
141/162 |
87 |
(80.9, 91.8) |
|
M. catarrhalis
|
22/26 |
84.6 |
(65.1, 95.6) |
|
|
15 to 18 Days Post-Therapy‡
(Secondary Endpoint) |
||
|
Pathogen
|
n/N
|
%
|
95% CI†
|
| All S. pneumoniae
|
95/136 |
69.9 |
(61.4, 77.4) |
|
S. pneumoniae with penicillin MIC = 2 mcg/mL |
11/20 |
55 |
(31.5, 76.9) |
|
S. pneumoniae with penicillin MIC = 4 mcg/mL |
5/14 |
35.7 |
(12.8, 64.9) |
|
H. influenzae
|
106/156 |
67.9 |
(60, 75.2) |
|
M. catarrhalis
|
14/25 |
56 |
(34.9, 75.6) |
S. pneumoniae
REFERENCES
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing – 21st Informational Supplement. CLSI Document M100-S21. CLSI, 940 West Valley Rd., Suite 1400, Wayne, PA 19087, 2011.
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria – Approved Standard 7th ed. CLSI Document M11-A7. CLSI, 940 West Valley Rd., Suite 1400, Wayne, PA 19087, 2007.
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard – 8th ed. CLSI Document M07-A8. CLSI, 940 West Valley Rd., Suite 1400, Wayne, PA19087, 2009.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Test; Approved Standard – 10th ed. CLSI Document M02-A10. CLSI, 940 West Valley Rd., Suite 1400, Wayne, PA 19087, 2009.
- Swanson-Biearman B, Dean BS, Lopez G, Krenzelok EP. The effects of penicillin and cephalosporin ingestions in children less than six years of age. Vet Hum Toxicol. 1988;30:66-67.
®
®
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 600 mg/42.9 mg per 5 mL (200 mL Bottle)
NDC 65862-535-02
Amoxicillin and Clavulanate
Potassium for Oral Suspension, USP
600 mg/42.9 mg* per 5 mL
Rx only
200 mL when reconstituted
AUROBINDO

Amoxicillin and Clavulanate PotassiumAmoxicillin and Clavulanate Potassium POWDER, FOR SUSPENSION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!