Amoxicillin and Clavulanate Potassium
Lake Erie Medical & Surgical Supply DBA Quality Care Products LLC
FULL PRESCRIBING INFORMATION: CONTENTS*
- AMOXICILLIN AND CLAVULANATE POTASSIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- AMOXICILLIN AND CLAVULANATE POTASSIUM INDICATIONS AND USAGE
- AMOXICILLIN AND CLAVULANATE POTASSIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- AMOXICILLIN AND CLAVULANATE POTASSIUM ADVERSE REACTIONS
- OVERDOSAGE
- AMOXICILLIN AND CLAVULANATE POTASSIUM DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- CLINICAL STUDIES
- REFERENCES
FULL PRESCRIBING INFORMATION
250 mg/125 mg
500 mg/125 mg
875 mg/125
mg
Rx only
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Amoxicillin and Clavulanate Potassium Tablets and other antibacterial drugs, Amoxicillin and Clavulanate Potassium Tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
AMOXICILLIN AND CLAVULANATE POTASSIUM DESCRIPTION
1619352SRRRp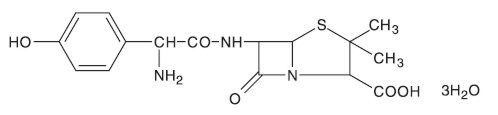
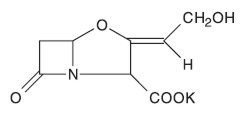
Streptomyces clavuligerus885ZRR
Inactive Ingredients: colloidal silicon dioxide, hypromellose, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, talc, titanium dioxide and triethyl citrate. In addition, the 250 mg/125 mg tablet contains cetyl alcohol and sodium lauryl sulphate; the 500 mg/125 mg tablet contains ethylcellulose aqueous dispersion; and the 875 mg/125 mg tablet contains crospovidone and ethylcellulose aqueous dispersion.
CLINICAL PHARMACOLOGY
*
|
Dose† and regimen |
AUC0-24 | (mcg∙hr/mL) | Cmax |
(mcg/mL)
|
|
amoxicillin/ clavulanate potassium |
amoxicillin (±S.D.) |
clavulante potassium (±S.D.) |
amoxicillin (±S.D.) |
clavulante potassium (±S.D.) |
| 250 mg/125 mg q8h |
26.7 ± 4.56 | 12.6 ± 3.25 | 3.3 ± 1.12 | 1.5 ± 0.70 |
| 500 mg/125 mg q12h |
33.4 ± 6.76 | 8.6 ± 1.95 | 6.5 ± 1.41 | 1.8 ± 0.61 |
| 500 mg/125 mg q8h |
53.4 ± 8.87 | 15.7 ± 3.86 | 7.2 ± 2.26 | 2.4 ± 0.83 |
| 875 mg/125 mg q12h |
53.5 ± 12.31 | 10.2 ± 3.04 | 11.6 ± 2.78 | 2.2 ± 0.99 |
†
Amoxicillin serum concentrations achieved with Amoxicillin and Clavulanate Potassium Tablets are similar to those produced by the oral administration of equivalent doses of amoxicillin alone. The half-life of amoxicillin after the oral administration of Amoxicillin and Clavulanate Potassium Tablets is 1.3 hours and that of clavulanic acid is 1 hour.
Approximately 50% to 70% of the amoxicillin and approximately 25% to 40% of the clavulanic acid are excreted unchanged in urine during the first 6 hours after administration of a single Amoxicillin and Clavulanate Potassium 250 mg/125 mg or 500 mg/125 mg Tablet.
Concurrent administration of probenecid delays amoxicillin excretion but does not delay renal excretion of clavulanic acid.
Neither component in Amoxicillin and Clavulanate Potassium Tablets is highly protein-bound; clavulanic acid has been found to be approximately 25% bound to human serum and amoxicillin approximately 18% bound.
Amoxicillin diffuses readily into most body tissues and fluids with the exception of the brain and spinal fluid. The results of experiments involving the administration of clavulanic acid to animals suggest that this compound, like amoxicillin, is well distributed in body tissues.
Amoxicillin is a semisynthetic antibiotic with a broad spectrum of bactericidal activity against many gram-positive and gram-negative microorganisms. Amoxicillin is, however, susceptible to degradation by β-lactamases, and therefore, the spectrum of activity does not include organisms which produce these enzymes. Clavulanic acid is a β-lactam, structurally related to the penicillins, which possesses the ability to inactivate a wide range of β-lactamase enzymes commonly found in microorganisms resistant to penicillins and cephalosporins. In particular, it has good activity against the clinically important plasmid-mediated β-lactamases frequently responsible for transferred drug resistance.
The formulation of amoxicillin and clavulanic acid in Amoxicillin and Clavulanate Potassium Tablets protects amoxicillin from degradation by β-lactamase enzymes and effectively extends the antibiotic spectrum of amoxicillin to include many bacteria normally resistant to amoxicillin and other β-lactam antibiotics. Thus, Amoxicillin and Clavulanate Potassium Tablets possess the properties of a broad-spectrum antibiotic and a β-lactamase inhibitor.
Amoxicillin/clavulanic acid has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in INDICATIONS AND USAGE.
Staphylococcus aureus (β-lactamase and non-β-lactamase-producing)1.
1
Enterobacter species (Although most
strains of Enterobacter species are resistant in vitro, clinical efficacy has been demonstrated with
Amoxicillin and Clavulanate Potassium Tablets in urinary tract infections caused
by these organisms.)
Escherichia coli (β-lactamase
and non-β-lactamase-producing)
Haemophilus
influenzae (β-lactamase and non-β-lactamase-producing)
Klebsiella species (All known strains are
β-lactamase-producing)
Moraxella catarrhalis
(β-lactamase and non-β-lactamase-producing)
The following in vitro data are available, but their clinical significance is unknown.
Amoxicillin/clavulanic acid exhibits in vitro minimal inhibitory concentrations (MICs) of 2 mcg/mL or less against most (≥90%) strains of Streptococcus pneumoniae 2; MICs of 0.06 mcg/mL or less against most (≥90%) strains of Neisseria gonorrhoeae; MICs of 4 mcg/mL or less against most (≥90%) strains of staphylococci and anaerobic bacteria; and MICs of 8 mcg/mL or less against most (≥90%) strains of other listed organisms. However, with the exception of organisms shown to respond to amoxicillin alone, the safety and effectiveness of amoxicillin/clavulanic acid in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
2in vitroS. pneumoniaeS. pneumoniae
Enterococcus faecalis
3
Staphylococcus epidermidis (β-lactamase and
non-β-lactamase-producing)
Staphylococcus
saprophyticus (β-lactamase and non-β-lactamase-producing)
Streptococcus pneumoniae
3/4
Streptococcus
pyogenes
3/4
viridans group Streptococcus
3/4
Eikenella corrodens (β-lactamase and
non-β-lactamase-producing)
Neisseria gonorrhoeae
3 (β-lactamase and
non-β-lactamase-producing)
Proteus mirabilis
3 (β-lactamase and
non-β-lactamase-producing)
Bacteroides species, including Bacteroides fragilis (β-lactamase and
non-β-lactamase-producing)
Fusobacterium species
(β-lactamase and non-β-lactamase-producing)
Peptostreptococcus species4
Quantitative methods are used to determine antimicrobial MICs. These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method1 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of amoxicillin/clavulanate potassium powder.
The recommended dilution pattern utilizes a constant amoxicillin/clavulanate potassium ratio of 2 to 1 in all tubes with varying amounts of amoxicillin. MICs are expressed in terms of the amoxicillin concentration in the presence of clavulanic acid at a constant 2 parts amoxicillin to 1 part clavulanic acid. The MIC values should be interpreted according to the following criteria:
|
For Gram-Negative
|
Enteric Aerobes:
|
|
|
|
MIC (mcg/mL) |
Interpretation
|
|
|
less than or equal to 8/4 |
Susceptible (S) |
|
|
16/8 |
Intermediate (I) |
|
|
greater than or equal to 32/16 |
Resistant (R) |
| For Staphylococcus * |
and
Haemophilus
species: |
|
|
|
MIC (mcg/mL) |
Interpretation
|
|
|
less than or equal to 4/2 |
Susceptible (S) |
|
|
greater than or equal to 8/4 |
Resistant (R) |
For S. pneumoniae from non-meningitis sources: Isolates should be tested using amoxicillin/clavulanic acid and the following criteria should be used:
| MIC (mcg/mL) | Interpretation |
| ≤2/1 | Susceptible (S) |
| 4/2 | Intermediate (I) |
| ≥8/4 | Resistant (R) |
Note: These interpretive criteria are based on the recommended doses for respiratory tract infections.
A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentration usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard amoxicillin/clavulanate potassium powder should provide the following MIC values:
| Microorganism | MIC Range (mcg/mL) * |
| Escherichia coli ATCC 25922 | 2 to 8 |
| Escherichia coli ATCC 35218 | 4 to 16 |
| Enterococcus faecalis ATCC 29212 | 0.25 to 1 |
| Haemophilus influenzae ATCC 49247 | 2 to 16 |
| Staphylococcus aureus ATCC 29213 | 0.12 to 0.5 |
| Streptococcus pneumoniae ATCC 49619 | 0.03 to 0.12 |
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 30 mcg of amoxicillin/clavulanate potassium (20 mcg amoxicillin plus 10 mcg clavulanate potassium) to test the susceptibility of microorganisms to amoxicillin/clavulanic acid.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30-mcg amoxicillin/clavulanate acid (20 mcg amoxicillin plus 10 mcg clavulanate potassium) disk should be interpreted according to the following criteria:
| For Staphylococcus * | species and H. influenzae †: |
|
|
|
Zone Diameter (mm)
|
Interpretation
|
|
|
greaer than or equal to 20 |
Susceptible (S) |
|
|
less than or equal to 19 |
Resistant (R) |
| For Other Organism | Except S. pneumoniae ‡ and N. gonorrhoeae §: |
|
|
|
Zone Diameter (mm) |
Interpretation
|
|
|
greater than or equal to 18 |
Susceptible (S) |
|
|
14 to 17 |
Intermediate (I) |
|
|
less than or equal to 13 |
Resistant (R) |
†H. influenzae.
‡S. pneumoniaeS. pneumoniae
§
N.gonorrhoeae
Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for amoxicillin/clavulanic acid.
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 30-mcg amoxicillin/clavulanate potassium (20-mcg amoxicillin plus 10-mcg clavulanate potassium) disk should provide the following zone diameters in these laboratory quality control strains:
| Microorganism | Zone Diameter (mm) |
| Escherichia coli ATCC 25922 | 19 to 25 |
| Escherichia coli ATCC 35218 | 18 to 22 |
| Staphylococcus aureus ATCC 25923 | 28 to 36 |
AMOXICILLIN AND CLAVULANATE POTASSIUM INDICATIONS AND USAGE
Amoxicillin and Clavulanate Potassium Tablets are indicated in the treatment of infections caused by susceptible strains of the designated organisms in the conditions listed below:
Lower Respiratory Tract Infections – caused by
β-lactamase-producing strains of H. influenzae and
M. catarrhalis.
Otitis Media
– caused by β-lactamase-producing strains of H.
influenzae and M. catarrhalis.
Sinusitis – caused by β-lactamase-producing strains of H. influenzae and M.
catarrhalis.
Skin and Skin Structure Infections
– caused by β-lactamase-producing strains of S.
aureus, E. coli, and Klebsiella spp.
Urinary Tract
Infections – caused by β-lactamase-producing strains of E. coli, Klebsiella spp., and
Enterobacter spp.
While Amoxicillin and Clavulanate Potassium Tablets are indicated only for the conditions listed above, infections caused by ampicillin-susceptible organisms are also amenable to treatment with Amoxicillin and Clavulanate Potassium Tablets due to its amoxicillin content; therefore, mixed infections caused by ampicillin-susceptible organisms and β-lactamase-producing organisms susceptible to Amoxicillin and Clavulanate Potassium Tablets should not require the addition of another antibiotic. Because amoxicillin has greater in vitro activity against S. pneumoniae than does ampicillin or penicillin, the majority of S. pneumoniae strains with intermediate susceptibility to ampicillin or penicillin are fully susceptible to amoxicillin and Amoxicillin and Clavulanate Potassium Tablets. (See Microbiology.)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Amoxicillin and Clavulanate Potassium Tablets and other antibacterial drugs, Amoxicillin and Clavulanate Potassium Tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Bacteriological studies, to determine the causative organisms and their susceptibility to Amoxicillin and Clavulanate Potassium Tablets, should be performed together with any indicated surgical procedures.
AMOXICILLIN AND CLAVULANATE POTASSIUM CONTRAINDICATIONS
Amoxicillin and Clavulanate Potassium Tablets are contraindicated in patients with a history of allergic reactions to any penicillin. It is also contraindicated in patients with a previous history of cholestatic jaundice/hepatic dysfunction associated with Amoxicillin and Clavulanate Potassium Tablets.
WARNINGS
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY AND/OR A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS. THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED WITH CEPHALOSPORINS. BEFORE INITIATING THERAPY WITH AMOXICILLIN AND CLAVULANATE POTASSIUM TABLETS, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS, OR OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, AMOXICILLIN AND CLAVULANATE POTASSIUM TABLETS SHOULD BE DISCONTINUED AND THE APPROPRIATE THERAPY INSTITUTED. SERIOUS ANAPHYLACTIC REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including Amoxicillin and Clavulanate Potassium Tablets, and has ranged in severity from mild to life-threatening; therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of "antibiotic-associated colitis."
After the diagnosis of pseudomembranous colitis has been established, appropriate therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against C. difficile colitis.
Amoxicillin and Clavulanate Potassium Tablets should be used with caution in patients with evidence of hepatic dysfunction. Hepatic toxicity associated with the use of Amoxicillin and Clavulanate Potassium Tablets is usually reversible. On rare occasions, deaths have been reported (less than 1 death reported per estimated 4 million prescriptions worldwide). These have generally been cases associated with serious underlying diseases or concomitant medications. (See CONTRAINDICATIONS and ADVERSE REACTIONS: Liver.)
PRECAUTIONS
While Amoxicillin and Clavulanate Potassium Tablets possess the characteristic low toxicity of the penicillin group of antibiotics, periodic assessment of organ system functions, including renal, hepatic, and hematopoietic function, is advisable during prolonged therapy.
A high percentage of patients with mononucleosis who receive ampicillin develop an erythematous skin rash. Thus, ampicillin-class antibiotics should not be administered to patients with mononucleosis.
The possibility of superinfections with mycotic or bacterial pathogens should be kept in mind during therapy. If superinfections occur (usually involving Pseudomonas or Candida), the drug should be discontinued and/or appropriate therapy instituted.
Prescribing Amoxicillin and Clavulanate Potassium Tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Probenecid decreases the renal tubular secretion of amoxicillin. Concurrent use with Amoxicillin and Clavulanate Potassium Tablets may result in increased and prolonged blood levels of amoxicillin. Coadministration of probenecid cannot be recommended.
The concurrent administration of allopurinol and ampicillin increases substantially the incidence of rashes in patients receiving both drugs as compared to patients receiving ampicillin alone. It is not known whether this potentiation of ampicillin rashes is due to allopurinol or the hyperuricemia present in these patients. There are no data with Amoxicillin and Clavulanate Potassium Tablets and allopurinol administered concurrently.
In common with other broad-spectrum antibiotics, Amoxicillin and Clavulanate Potassium Tablets may reduce the efficacy of oral contraceptives.
Oral administration of Amoxicillin and Clavulanate Potassium Tablets will result in high urine concentrations of amoxicillin. High urine concentrations of ampicillin may result in false-positive reactions when testing for the presence of glucose in urine using Clinitest®, Benedict's Solution, or Fehling's Solution. Since this effect may also occur with amoxicillin and therefore Amoxicillin and Clavulanate Potassium Tablets, it is recommended that glucose tests based on enzymatic glucose oxidase reactions (such as Clinistix®) be used.
Following administration of ampicillin to pregnant women, a transient decrease in plasma concentration of total conjugated estriol-estriol-glucuronide, conjugated estrone and estradiol has been noted. This effect may also occur with amoxicillin and therefore Amoxicillin and Clavulanate Potassium Tablets.
Patients should be counseled that antibacterial drugs including Amoxicillin and Clavulanate Potassium Tablets, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Amoxicillin and Clavulanate Potassium Tablets are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of the immediate treatment, and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Amoxicillin and Clavulanate Potassium Tablets or other antibacterial drugs in the future.
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
The mutagenic potential of Amoxicillin and Clavulanate Potassium Tablets was investigated in vitro with an Ames test, a human lymphocyte cytogenetic assay, a yeast test and a mouse lymphoma forward mutation assay, and in vivo with mouse micronucleus tests and a dominant lethal test. All were negative apart from the in vitro mouse lymphoma assay where weak activity was found at very high, cytotoxic concentrations.
Amoxicillin and Clavulanate Potassium Tablets at oral doses of up to 1,200 mg/kg/day (5.7 times the maximum human dose, 1,480 mg/m2/day, based on body surface area) was found to have no effect on fertility and reproductive performance in rats, dosed with a 2:1 ratio formulation of amoxicillin:clavulanate.
Reproduction studies performed in pregnant rats and mice given Amoxicillin and Clavulanate Potassium Tablets at oral dosages up to 1,200 mg/kg/day, equivalent to 7,200 and 4,080 mg/m2/day, respectively (4.9 and 2.8 times the maximum human oral dose based on body surface area), revealed no evidence of harm to the fetus due to Amoxicillin and Clavulanate Potassium Tablets. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Oral ampicillin-class antibiotics are generally poorly absorbed during labor. Studies in guinea pigs have shown that intravenous administration of ampicillin decreased the uterine tone, frequency of contractions, height of contractions, and duration of contractions; however, it is not known whether the use of Amoxicillin and Clavulanate Potassium Tablets in humans during labor or delivery has immediate or delayed adverse effects on the fetus, prolongs the duration of labor, or increases the likelihood that forceps delivery or other obstetrical intervention or resuscitation of the newborn will be necessary. In a single study in women with premature rupture of fetal membranes, it was reported that prophylactic treatment with Amoxicillin and Clavulanate Potassium Tablets may be associated with an increased risk of necrotizing enterocolitis in neonates.
Ampicillin-class antibiotics are excreted in the milk; therefore, caution should be exercised when Amoxicillin and Clavulanate Potassium Tablets are administered to a nursing woman.
Pediatric patients weighing 40 kg or more should be dosed according to the adult recommendations (see DOSAGE AND ADMINISTRATION: Pediatric Patients ). Safety and effectiveness of Amoxicillin and Clavulanate Potassium Tablets in pediatric patients weighing less than 40 kg have not been established. (See prescribing information for Amoxicillin and Clavulanate Potassium Powder for Oral Suspension and Chewable Tablets.)
An analysis of clinical studies of Amoxicillin and Clavulanate Potassium Tablets was conducted to determine whether subjects aged 65 and over respond differently from younger subjects. Of the 3,119 patients in this analysis, 68% were less than 65 years old, 32% were greater than or equal to 65 years old and 14% were greater than or equal to 75 years old. This analysis and other reported clinical experience have not identified differences in responses between the elderly and younger patients, but a greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
AMOXICILLIN AND CLAVULANATE POTASSIUM ADVERSE REACTIONS
Amoxicillin and Clavulanate Potassium Tablets are generally well tolerated. The majority of side effects observed in clinical trials were of a mild and transient nature and less than 3% of patients discontinued therapy because of drug-related side effects. The most frequently reported adverse effects were diarrhea/loose stools (9%), nausea (3%), skin rashes and urticaria (3%), vomiting (1%) and vaginitis (1%). The overall incidence of side effects, and in particular diarrhea, increased with the higher recommended dose. Other less frequently reported reactions include: Abdominal discomfort, flatulence, and headache.
The following adverse reactions have been reported for ampicillin-class antibiotics:
Diarrhea, nausea, vomiting, indigestion, gastritis, stomatitis, glossitis, black "hairy" tongue, mucocutaneous candidiasis, enterocolitis, and hemorrhagic/pseudomembranous colitis. Onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment. (See WARNINGS.)
Skin rashes, pruritus, urticaria, angioedema, serum sickness-like reactions (urticaria or skin rash accompanied by arthritis, arthralgia, myalgia, and frequently fever), erythema multiforme (rarely Stevens-Johnson syndrome), acute generalized exanthematous pustulosis, and an occasional case of exfoliative dermatitis (including toxic epidermal necrolysis) have been reported. These reactions may be controlled with antihistamines and, if necessary, systemic corticosteroids. Whenever such reactions occur, the drug should be discontinued, unless the opinion of the physician dictates otherwise. Serious and occasional fatal hypersensitivity (anaphylactic) reactions can occur with oral penicillin. (See WARNINGS.)
A moderate rise in AST (SGOT) and/or ALT (SGPT) has been noted in patients treated with ampicillin-class antibiotics but the significance of these findings is unknown. Hepatic dysfunction, including increases in serum transaminases (AST and/or ALT), serum bilirubin, and/or alkaline phosphatase, has been infrequently reported with Amoxicillin and Clavulanate Potassium Tablets. It has been reported more commonly in the elderly, in males, or in patients on prolonged treatment. The histologic findings on liver biopsy have consisted of predominantly cholestatic, hepatocellular, or mixed cholestatic-hepatocellular changes. The onset of signs/symptoms of hepatic dysfunction may occur during or several weeks after therapy has been discontinued. The hepatic dysfunction, which may be severe, is usually reversible. On rare occasions, deaths have been reported (less than 1 death reported per estimated 4 million prescriptions worldwide). These have generally been cases associated with serious underlying diseases or concomitant medications.
Interstitial nephritis and hematuria have been reported rarely. Crystalluria has also been reported (See OVERDOSAGE ).
Anemia, including hemolytic anemia, thrombocytopenia, thrombocytopenic purpura, eosinophilia, leukopenia and agranulocytosis have been reported during therapy with penicillins. These reactions are usually reversible on discontinuation of therapy and are believed to be hypersensitivity phenomena. A slight thrombocytosis was noted in less than 1% of the patients treated with Amoxicillin and Clavulanate Potassium Tablets. There have been reports of increased prothrombin time in patients receiving Amoxicillin and Clavulanate Potassium Tablets and anticoagulant therapy concomitantly.
Agitation, anxiety, behavioral changes, confusion, convulsions, dizziness, insomnia, and reversible hyperactivity have been reported rarely.
Tooth discoloration (brown, yellow, or gray staining) has been rarely reported. Most reports occured in pediatric patients. Discoloration was reduced or eliminated with brushing or dental cleaning in most cases.
OVERDOSAGE
Following overdosage, patients have experienced primarily gastrointestinal symptoms including stomach and abdominal pain, vomiting, and diarrhea. Rash, hyperactivity, or drowsiness have also been observed in a small number of patients.
In the case of overdosage, discontinue Amoxicillin and Clavulanate Potassium Tablets, treat symptomatically, and institute supportive measures as required. If the overdosage is very recent and there is no contraindication, an attempt at emesis or other means of removal of drug from the stomach may be performed. A prospective study of 51 pediatric patients at a poison center suggested that overdosages of less than 250 mg/kg of amoxicillin are not associated with significant clinical symptoms and do not require gastric emptying3.
Interstitial nephritis resulting in oliguric renal failure has been reported in a small number of patients after overdosage with amoxicillin.
Crystalluria, in some cases leading to renal failure, has also been reported after amoxicillin overdosage in adult and pediatric patients. In case of overdosage, adequate fluid intake and diuresis should be maintained to reduce the risk of amoxicillin crystalluria.
Renal impairment appears to be reversible with cessation of drug administration. High blood levels may occur more readily in patients with impaired renal function because of decreased renal clearance of both amoxicillin and clavulanate. Both amoxicillin and clavulanate are removed from the circulation by hemodialysis. (See DOSAGE AND ADMINISTRATION for recommended dosing for patients with impaired renal function.)
AMOXICILLIN AND CLAVULANATE POTASSIUM DOSAGE AND ADMINISTRATION
Since both the Amoxicillin and Clavulanate Potassium 250 mg/125 mg and 500 mg/125 mg Tablets contain the same amount of clavulanic acid (125 mg, as the potassium salt), two Amoxicillin and Clavulanate Potassium 250 mg/125 mg Tablets are not equivalent to one Amoxicillin and Clavulanate Potassium 500 mg/125 mg Tablet; therefore, two Amoxicillin and Clavulanate Potassium 250 mg/125 mg Tablets should not be substituted for one Amoxicillin and Clavulanate Potassium 500 mg/125 mg Tablet.
The usual adult dose is one Amoxicillin and Clavulanate Potassium 500 mg/125 mg Tablet every 12 hours or one Amoxicillin and Clavulanate Potassium 250 mg/125 mg Tablet every 8 hours. For more severe infections and infections of the respiratory tract, the dose should be one Amoxicillin and Clavulanate Potassium 875 mg/125 mg Tablet every 12 hours or one Amoxicillin and Clavulanate Potassium 500 mg/125 mg Tablet every 8 hours.
Patients with impaired renal function do not generally require a reduction in dose unless the impairment is severe. Severely impaired patients with a glomerular filtration rate of less than 30 mL/min. should not receive the 875 mg/125 mg tablet. Patients with a glomerular filtration rate of 10 to 30 mL/ min. should receive 500 mg/125 mg or 250 mg/125 mg every 12 hours, depending on the severity of the infection. Patients with a less than 10 mL/min. glomerular filtration rate should receive 500 mg/125 mg or 250 mg/125 mg every 24 hours, depending on severity of the infection.
Hemodialysis patients should receive 500 mg/125 mg or 250 mg/125 mg every 24 hours, depending on severity of the infection. They should receive an additional dose both during and at the end of dialysis.
Hepatically impaired patients should be dosed with caution and hepatic function monitored at regular intervals. (See WARNINGS.)
Pediatric patients weighing 40 kg or more should be dosed according to the adult recommendations.
Due to the different amoxicillin to clavulanic acid ratios in the Amoxicillin and Clavulanate Potassium Tablets (250 mg/125 mg) versus the Amoxicillin and Clavulanate Potassium Chewable Tablets (250 mg/62.5 mg), the Amoxicillin and Clavulanate Potassium 250 mg/125 mg Tablets should not be used until the pediatric patient weighs at least 40 kg or more.
Administration: Amoxicillin and Clavulanate Potassium Tablets may be taken without regard to meals; however, absorption of clavulanate potassium is enhanced when Amoxicillin and Clavulanate Potassium Tablets are administered at the start of a meal. To minimize the potential for gastrointestinal intolerance, Amoxicillin and Clavulanate Potassium Tablets should be taken at the start of a meal.
HOW SUPPLIED
Amoxicillin and Clavulanate Potassium Tablets, USP, 500 mg/125 mg: Each film coated tablet, for oral administration, is white, oval-shaped, debossed GGN6 on one side and plain on the reverse side, and contains 500 mg amoxicillin as the trihydrate and 125 mg clavulanic acid as the potassium salt.
NDC 54868-4743-2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
bottles of 15
NDC 54868-4743-0 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
bottles of 20
NDC 54868-4743-1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
bottles of 30
Amoxicillin and Clavulanate Potassium Tablets, USP, 875 mg/125 mg: Each film coated tablet, for oral administration, is white, capsule-shaped, scored and debossed GGN7 on one side and scored on the reverse side, and contains 875 mg amoxicillin as the trihydrate and 125 mg clavulanic acid as the potassium salt.
NDC 54868-4951-0 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
bottles of 10
NDC 54868-4951-2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
bottles of 14
NDC 54868-4951-1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
bottles of 20
NDC 54868-4951-4 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
bottles of 28
NDC 54868-4951-3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
bottles of 30
Store at 20°-25 °C (68°-77°F) [See USP Controlled Room Temperature].
Dispense in tightly closed, moisture-proof containers.
CLINICAL STUDIES
Data from two pivotal studies in 1,191 patients treated for either lower respiratory tract infections or complicated urinary tract infections compared a regimen of 875 mg/125 mg Amoxicillin and Clavulanate Potassium Tablets q12h to 500 mg/125 mg Amoxicillin and Clavulanate Potassium Tablets dosed q8h (584 and 607 patients, respectively). Comparable efficacy was demonstrated between the q12h and q8h dosing regimens. There was no significant difference in the percentage of adverse events in each group. The most frequently reported adverse event was diarrhea; incidence rates were similar for the 875 mg/125 mg q12h and 500 mg/125 mg q8h dosing regimens (14.9% and 14.3%, respectively); however, there was a statistically significant difference (p less than 0.05) in rates of severe diarrhea or withdrawals with diarrhea between the regimens: 1% for 875 mg/125 mg q12h dosing versus 2.5% for the 500 mg/125 mg q8h dosing.
In 1 of these pivotal studies, 629 patients with either pyelonephritis or a complicated urinary tract infection (i.e., patients with abnormalities of the urinary tract that predispose to relapse of bacteriuria following eradication) were randomized to receive either 875 mg/125 mg Amoxicillin and Clavulanate Potassium Tablets q12h or 500 mg/125 mg Amoxicillin and Clavulanate Potassium Tablets q8h in the following distribution:
|
|
875 mg/125 mg q12h | 500 mg/125 mg q8h |
|---|---|---|
| Pyelonephritis | 173 patients | 188 patients |
| Complicated UTI | 135 patients | 133 patients |
| Total patients | 308 | 321 |
The number of bacteriologically evaluable patients was comparable between the two dosing regimens. Amoxicillin and Clavulanate Potassium Tablets produced comparable bacteriological success rates in patients assessed 2 to 4 days immediately following end of therapy. The bacteriological efficacy rates were comparable at one of the follow-up visits (5 to 9 days post-therapy) and at a late post-therapy visit (in the majority of cases, this was 2 to 4 weeks post-therapy), as seen in the table below:
|
|
875 mg/125 mg q12h | 500 mg/125 mg q8h |
|---|---|---|
| 2 to 4 days | 81%, n=58 | 80%, n=54 |
| 5 to 9 days | 58.5%, n=41 | 51.9%, n=52 |
| 2 to 4 weeks | 52.5%, n=101 | 54.8%, n=104 |
As noted before, though there was no significant difference in the percentage of adverse events in each group, there was a statistically sinificant difference in rates of severe diarrhea or withdrawals with diarrhea between the regimens.
REFERENCES
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically - Third Edition. Approved Standard NCCLS Document M7-A3, Vol. 13, No. 25. NCCLS, Villanova, PA, December 1993.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests - Fifth Edition. Approved Standard NCCLS Document M2-A5, Vol. 13, No. 24. NCCLS, Villanova, PA, December 1993.
- Swanson-Biearman B, Dean BS, Lopez G, Krenzelok EP. The effects of penicillin and cephalosporin ingestions in children less than six years of age. Vet Hum Toxicol 1988; 30: 66-67.
CLINITEST® is a registered trademark of
Miles, Inc.
CLINISTIX® is a registered trademark of
Bayer Corporation.
Revised: June 2007
8083
8038
8039
Manufactured in Austria by Sandoz GmbH
for Sandoz Inc., Princeton, NJ
08540
image of label
Amoxicillin and Clavulanate PotassiumAmoxicillin and Clavulanate Potassium TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||