Amoxicillin
Amoxicillin Capsules, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- AMOXICILLIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- AMOXICILLIN INDICATIONS AND USAGE
- AMOXICILLIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- AMOXICILLIN ADVERSE REACTIONS
- OVERDOSAGE
- AMOXICILLIN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- CLINICAL STUDIES
- REFERENCES
FULL PRESCRIBING INFORMATION
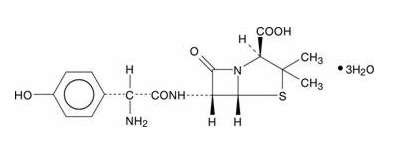
AMOXICILLIN DESCRIPTION
SRRRp
1619352
CLINICAL PHARMACOLOGY
0-∞max
| * Administered at the start of a light meal. † Mean values of 24 normal volunteers. Peak concentrations occurred approximately 1 hour after the dose. |
||
| Dose* |
AUC0-∞ (mcg•hr/mL) |
Cmax (mcg/mL)† |
| Amoxicillin |
Amoxicillin (±S.D.) |
Amoxicillin (±S.D.) |
| 400 mg (5 mL of suspension) |
17.1 (3.1) |
5.92 (1.62) |
| 400 mg (1 chewable tablet) |
17.9 (2.4) |
5.18 (1.64) |
Microbiology
in vitro INDICATIONS AND USAGE
Aerobic Gram-Positive Microorganisms
Enterococcus faecalis
Staphylococcus
Streptococcus pneumoniae
Streptococcus
Aerobic Gram-Negative Microorganisms
Escherichia coli
Haemophilus influenzae
Neisseria gonorrhoeae
Proteus mirabilis
Helicobacter
Helicobacter pylori
Susceptibility Tests
Dilution Techniques
1ampicillinS. pneumoniaeS. pneumoniae
Enterococcus
| MIC (mcg/mL)
|
Interpretation
|
| ≤8
|
Susceptible (S)
|
| ≥16
|
Resistant (R)
|
Staphylococcusa
| MIC (mcg/mL)
|
Interpretation
|
| ≤0.25
|
Susceptible (S)
|
| ≥0.5
|
Resistant (R)
|
Streptococcus S. pneumoniae
| MIC (mcg/mL)
|
Interpretation
|
| ≤0.25
|
Susceptible (S)
|
| 0.5 to 4
|
Intermediate (I)
|
| ≥8 |
Resistant (R) |
S. pneumoniaeb
Amoxicillin
| MIC (mcg/mL)
|
Interpretation
|
| ≤2
|
Susceptible (S)
|
| 4
|
Intermediate (I)
|
| ≥8 |
Resistant (R) |
NOTE:
| MIC (mcg/mL)
|
Interpretation
|
| ≤8
|
Susceptible (S)
|
| 16
|
Intermediate (I)
|
| ≥32 |
Resistant (R) |
H. influenzaec
| MIC (mcg/mL)
|
Interpretation
|
| ≤1
|
Susceptible (S)
|
| 2
|
Intermediate (I)
|
| ≥4 |
Resistant (R) |
H. influenzae Haemophilus1
ampicillin
|
Microorganism
|
MIC Range (mcg/mL)
|
|
E. coli ATCC 25922
|
2 to 8 |
|
E. faecalis ATCC 29212
|
0.5 to 2 |
|
H. influenzae ATCC 49247d
|
2 to 8 |
|
S. aureus ATCC 29213
|
0.25 to 1 |
amoxicillin
|
Microorganism
|
MIC Range (mcg/mL)
|
|
S. pneumoniae ATCC 49619e
|
0.03 to 0.12 |
H. influenzae1
S. pneumoniae
Diffusion Techniques
2S. pneumoniaeampicillin
Enterococcus
| Zone Diameter (mm)
|
Interpretation
|
| ≥17 |
Susceptible (S) |
| ≤16 |
Resistant (R) |
Staphylococcusf
| Zone Diameter (mm)
|
Interpretation
|
| ≥29 |
Susceptible (S) |
| ≤28 |
Resistant (R) |
-
|
Zone Diameter (mm)
|
Interpretation
|
| ≥26 |
Susceptible (S) |
| 19 to 25 |
Intermediate (I) |
| ≤18 |
Resistant (R) |
NOTE:Spneumoniae
S. pneumoniae
S. pneumoniae S. pneumoniae
Enterobacteriaceae
| Zone Diameter (mm)
|
Interpretation
|
| ≥17 |
Susceptible (S) |
| 14 to 16 |
Intermediate (I) |
| ≤13 |
Resistant (R) |
H. influenzaeg
| Zone Diameter (mm)
|
Interpretation
|
| ≥22 |
Susceptible (S) |
| 19 to 21 |
Intermediate (I) |
| ≤18 |
Resistant (R) |
H. influenzaeHaemophilus2
ampicillin
|
Microorganism
|
Zone Diameter (mm)
|
|
E. coli ATCC 25922
|
16 to 22 |
|
H. influenzae ATCC 49247h
|
13 to 21 |
|
S. aureus ATCC 25923 |
27 to 35 |
oxacillin
|
Microorganism
|
Zone Diameter (mm)
|
|
S. pneumoniae ATCC 49619i
|
8 to 12 |
H. influenzae2
S. pneumoniae2
Susceptibility Testing for Helicobacter pylori
In vitroH. pylori
AMOXICILLIN INDICATIONS AND USAGE
Infections of the ear, nose, and throatStreptococcusS. pneumoniaeStaphylococcusH. influenzae
Infections of the genitourinary tractE. coli, P. mirabilisE. faecalis
Infections of the skin and skin structureStreptococcusStaphylococcusE. coli
Infections of the lower respiratory tractStreptococcusS. pneumoniae, StaphylococcusH. influenzae
Gonorrhea, acute uncomplicated (ano-genital and urethral infections)N. gonorrhoeae
H. pylori eradication to reduce the risk of duodenal ulcer recurrence
Triple Therapy
Amoxicillin/clarithromycin/lansoprazole
H. pyloriH. pyloriH. pylori CLINICAL STUDIES DOSAGE AND ADMINISTRATION
Dual Therapy
Amoxicillin/lansoprazole
H. pyloriwho are either allergic or intolerant to clarithromycin or in whom resistance to clarithromycin is known or suspected.H. pylori CLINICAL STUDIES DOSAGE AND ADMINISTRATION
AMOXICILLIN CONTRAINDICATIONS
WARNINGS
SERIOUS ANAPHYLACTIC REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS, AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Clostridium difficile C. difficile.
C. difficile C. difficile
C. difficileC. difficile
PRECAUTIONS
General
Laboratory Tests
Drug Interactions
in vitro
Drug/Laboratory Test Interactions
®®
Carcinogenesis, Mutagenesis, Impairment Of Fertility
2
Pregnancy
Teratogenic Effects
Labor and Delivery
Nursing Mothers
Pediatric Use
DOSAGE AND ADMINISTRATION: Neonates and Infants
Geriatric Use
Information for Patients
AMOXICILLIN ADVERSE REACTIONS
Infections and Infestations
Gastrointestinal
WARNINGS
Hypersensitivity Reactions
WARNINGS
NOTE:
Liver
Renal
OVERDOSAGE
Hemic and Lymphatic Systems
Central Nervous System
Miscellaneous
Combination Therapy with Clarithromycin and Lansoprazole
Triple Therapy
Amoxicillin/Clarithromycin/Lansoprazole
Dual Therapy
Amoxicillin/Lansoprazole
OVERDOSAGE
3
AMOXICILLIN DOSAGE AND ADMINISTRATION
Neonates and Infants Aged ≤12 Weeks (≤3 Months)
Adults and Pediatric Patients >3 Months
| * Dosing for infections caused by less susceptible organisms should follow the recommendations for severe infections. † The children’s dosage is intended for individuals whose weight is less than 40 kg. Children weighing 40 kg or more should be dosed according to the adult recommendations. ‡ Each strength of the suspension of amoxicillin is available as a chewable tablet for use by older children. |
|||
| Infection |
Severity* |
Usual Adult Dose |
Usual Dose for Children >3 Months†‡ |
| Ear/Nose/Throat |
Mild/Moderate |
500 mg every 12 hours or 250 mg every 8 hours |
25 mg/kg/day in divided doses every 12 hours or 20 mg/kg/day in divided doses every 8 hours |
| Severe |
875 mg every 12 hours or 500 mg every 8 hours |
45 mg/kg/day in divided doses every 12 hours or 40 mg/kg/day in divided doses every 8 hours |
|
| Lower Respiratory Tract |
Mild/Moderate or Severe |
875 mg every 12 hours or 500 mg every 8 hours |
45 mg/kg/day in divided doses every 12 hours or 40 mg/kg/day in divided doses every 8 hours |
| Skin/Skin Structure |
Mild/Moderate |
500 mg every 12 hours or 250 mg every 8 hours |
25 mg/kg/day in divided doses every 12 hours or 20 mg/kg/day in divided doses every 8 hours |
| Severe |
875 mg every 12 hours or 500 mg every 8 hours |
45 mg/kg/day in divided doses every 12 hours or 40 mg/kg/day in divided doses every 8 hours |
|
| Genitourinary Tract |
Mild/Moderate |
500 mg every 12 hours or 250 mg every 8 hours |
25 mg/kg/day in divided doses every 12 hours or 20 mg/kg/day in divided doses every 8 hours |
| Severe |
875 mg every 12 hours or 500 mg every 8 hours |
45 mg/kg/day in divided doses every 12 hours or 40 mg/kg/day in divided doses every 8 hours |
|
| Gonorrhea Acute, uncomplicated ano-genital and urethral infections in males and females |
|
3 grams as single oral dose |
Prepubertal children: 50 mg/kg amoxicillin, combined with 25 mg/kg probenecid as a single dose. NOTE: SINCE PROBENECID IS CONTRAINDICATED IN CHILDREN UNDER 2 YEARS, DO NOT USE THIS REGIMEN IN THESE CASES. |
PRECAUTIONS: Laboratory Tests
General
Streptococcus pyogenes
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
Triple Therapy
Amoxicillin/clarithromycin/lansoprazole
INDICATIONS AND USAGE
Dual Therapy
Amoxicillin/lansoprazole
INDICATIONS AND USAGE
Dosing Recommendations for Adults with Impaired Renal Function
There are currently no dosing recommendations for pediatric patients with impaired renal function.
HOW SUPPLIED
Capsules
Amoxicillin Capsules, USP
250 mg Capsule
500 mg Capsule
Store at
CLINICAL STUDIES
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
H. pyloriH. pylori
Triple Therapy
Dual Therapy
H. pylori
H. pylori
| * This analysis was based on evaluable patients with confirmed duodenal ulcer (active or within 1 year) and H. pylori infection at baseline defined as at least 2 of 3 positive endoscopic tests from CLOtest®, (Delta West Ltd., Bentley, Australia), histology, and/or culture. Patients were included in the analysis if they completed the study. Additionally, if patients dropped out of the study due to an adverse event related to the study drug, they were included in the analysis as failures of therapy. † Patients were included in the analysis if they had documented H. pylori infection at baseline as defined above and had a confirmed duodenal ulcer (active or within 1 year). All dropouts were included as failures of therapy. ‡ (p<0.05) versus lansoprazole/amoxicillin and lansoprazole/clarithromycin dual therapy. § (p<0.05) versus clarithromycin/amoxicillin dual therapy. |
||
| Study |
Triple Therapy |
Triple Therapy |
| Evaluable Analysis*
|
Intent-to-Treat Analysis†
|
|
| Study 1 |
92‡
[80 - 97.7] (n = 48) |
86‡
[73.3 - 93.5] (n = 55) |
| Study 2 |
86§
[75.7 - 93.6] (n = 66) |
83§
[72 - 90.8] (n = 70) |
| * This analysis was based on evaluable patients with confirmed duodenal ulcer (active or within 1 year) and H. pylori infection at baseline defined as at least 2 of 3 positive endoscopic tests from CLOtest®, histology, and/or culture. Patients were included in the analysis if they completed the study. Additionally, if patients dropped out of the study due to an adverse event related to the study drug, they were included in the analysis as failures of therapy. † Patients were included in the analysis if they had documented H. pylori infection at baseline as defined above and had a confirmed duodenal ulcer (active or within 1 year). All dropouts were included as failures of therapy. ‡ (p<0.05) versus lansoprazole alone. § (p<0.05) versus lansoprazole alone or amoxicillin alone. |
||
| Study |
Dual Therapy |
Dual Therapy |
| Evaluable Analysis* |
Intent-to-Treat Analysis†
|
|
| Study 1 |
77‡
[62.5 - 87.2] (n = 51) |
70‡
[56.8 - 81.2] (n = 60) |
| Study 2 |
66§
[51.9 - 77.5] (n = 58) |
61§
[48.5 - 72.9] (n = 67) |
REFERENCES
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically – Fourth Edition; Approved Standard NCCLS Document M7-A4, Vol. 17, No. 2. NCCLS, Wayne, PA, January 1997.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests – Sixth Edition; Approved Standard NCCLS Document M2-A6, Vol. 17, No. 1. NCCLS, Wayne, PA, January 1997.
- Swanson-Biearman B, Dean BS, Lopez G, Krenzelok EP. The effects of penicillin and cephalosporin ingestions in children less than six years of age. Vet Hum Toxicol. 1988;30:66-67.
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited

AmoxicillinAmoxicillin CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!