AMOREPACIFIC
AMOREPACIFIC Time Response Skin Renewal Foundation SPF 18
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS
- PURPOSE
- USE
- WARNINGS
- INACTIVE INGREDIENTS
- PRINCIPAL DISPLAY PANEL - 30 ml Carton (102)
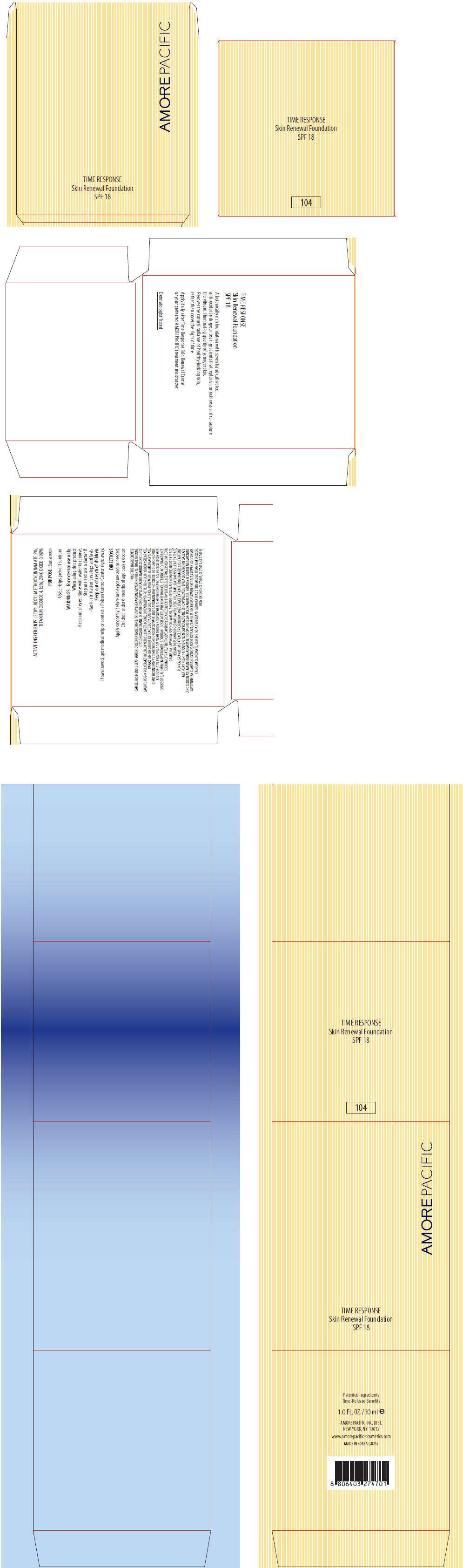
- PRINCIPAL DISPLAY PANEL - 30 ml Carton (104)
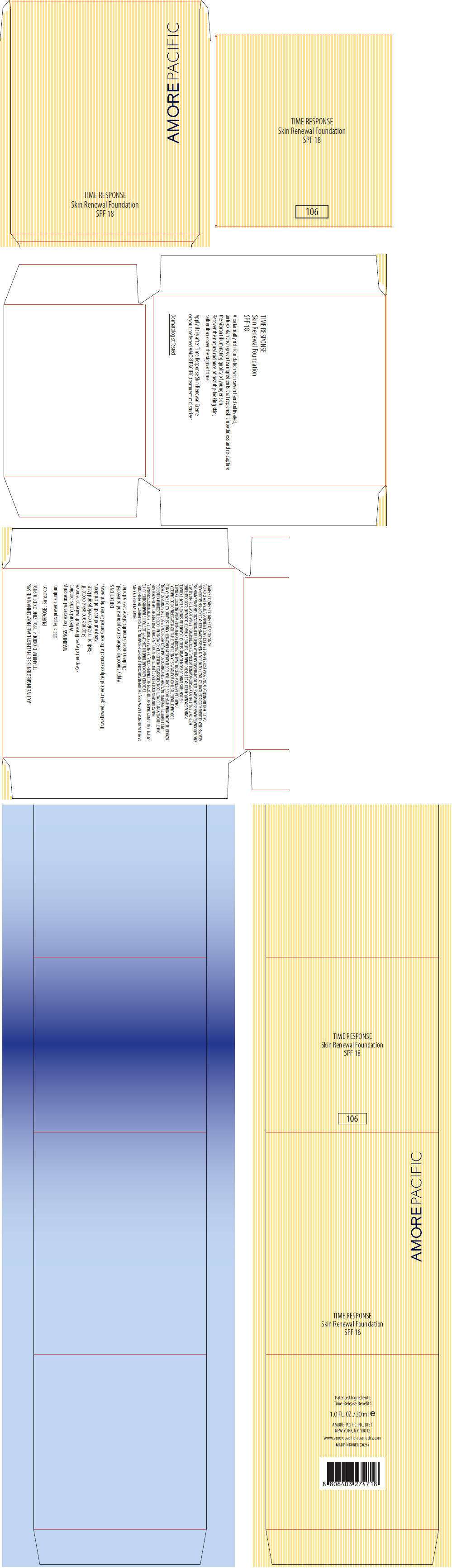
- PRINCIPAL DISPLAY PANEL - 30 ml Carton (106)
- PRINCIPAL DISPLAY PANEL - 30 ml Carton (202)
- PRINCIPAL DISPLAY PANEL - 30 ml Carton (204)
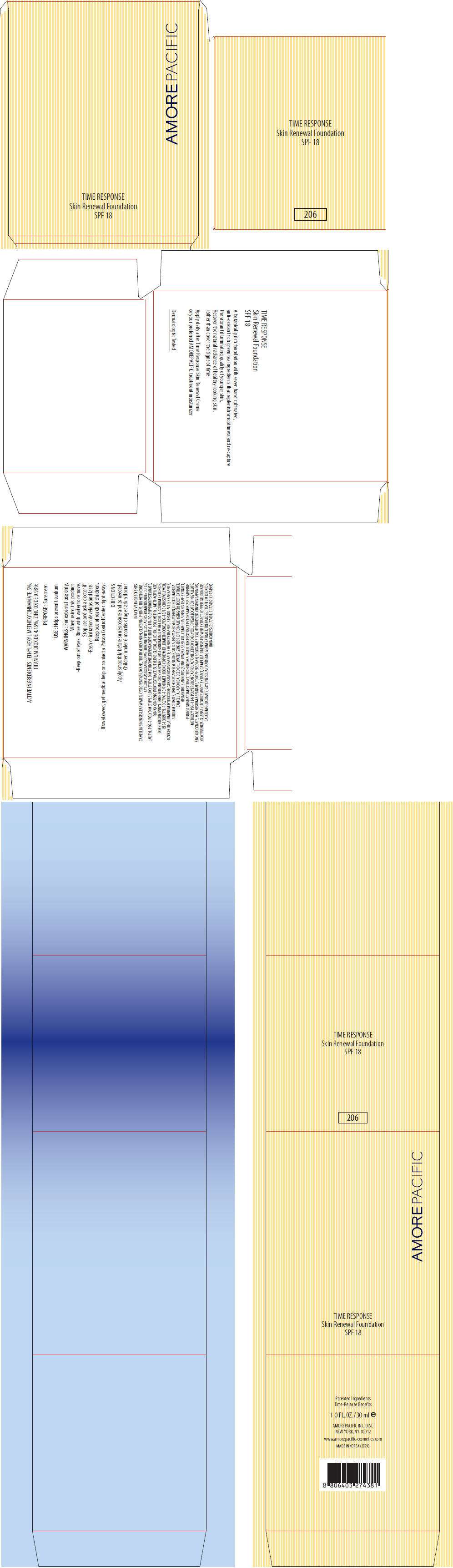
- PRINCIPAL DISPLAY PANEL - 30 ml Carton (206)
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS
ETHYLHEXYL METHOXYCINNAMATE 5%, TITANIUM DIOXIDE 4.15%, ZINC OXIDE 0.98%
PURPOSE
Sunscreen
USE
Helps prevent sunburn
WARNINGS
For external use only.
When using this product
-Keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
-Rash or irritation develops and lasts
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
Apply smoothly before sun exposure and as needed.
Children under 6 months of age : ask a doctor
INACTIVE INGREDIENTS
CAMELLIA SINENSIS LEAF WATER, CYCLOPENTASILOXANE, TRIETHYLHEXANOIN, GLYCERIN, PHENYL TRIMETHICONE, CYCLOHEXASILOXANE, DIMETHICONE, PHYLLOSTACHIS BAMBUSOIDES JUICE, LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, DIPENTAERYTHRITYL TRI-POLYHYDROXYSTEARATE, PANAX GINSENG ROOT EXTRACT, BUTYLENE GLYCOL, ALCOHOL, POLYMETHYL METHACRYLATE, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, DISTEARDIMONIUM HECTORITE, SODIUM CHLORIDE, BIS-ISOBUTYL PEG/PPG-10/7/DIMETHICONE COPOLYMER, DIMETHICONE/PEG-10/15 CROSSPOLYMER, OZOKERITE, ALUMINUM HYDROXIDE, SORBITAN ISOSTEARATE, PHENOXYETHANOL, STEARIC ACID, FRAGRANCE, SODIUM CITRATE, TRIETHOXYCAPRYLYLSILANE, SILICA, ETHYLHEXYLGLYCERIN, DISODIUM EDTA, CAMELLIA JAPONICA SEED OIL, WATER, ZINGIBER OFFICINALE (GINGER) ROOT EXTRACT, HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL, CAMELLIA SINENSIS LEAF EXTRACT, PUNICA GRANATUM EXTRACT, TRICHOLOMA MATSUTAKE EXTRACT, POLOXAMER 235, CAFFEINE, METHOXY PEG-114/POLYEPSILON CAPROLACTONE, ETHOXYDIGLYCOL, EPIGALLOCATECHIN GALLATE, ZINC GLUCONATE, MAGNESIUM ASPARTATE, TOCOPHERSOLAN, POLOXAMER 338, HYDROLYZED GINSENG SAPONINS, GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT, CAMELLIA SINENSIS FLOWER EXTRACT, COPPER GLUCONATE, CALCIUM GLUCONATE, GLYCINE SOJA (SOYBEAN) GERM EXTRACT, TREHALOSE, TITANIUM DIOXIDE, IRON OXIDES (CI 77491, CI 77492, CI 77499)
PRINCIPAL DISPLAY PANEL - 30 ml Carton (102)
AMOREPACIFIC
TIME RESPONSE
Skin Renewal Foundation
SPF 18

PRINCIPAL DISPLAY PANEL - 30 ml Carton (104)
AMOREPACIFIC
TIME RESPONSE
Skin Renewal Foundation
SPF 18
PRINCIPAL DISPLAY PANEL - 30 ml Carton (106)
AMOREPACIFIC
TIME RESPONSE
Skin Renewal Foundation
SPF 18
PRINCIPAL DISPLAY PANEL - 30 ml Carton (202)
AMOREPACIFIC
TIME RESPONSE
Skin Renewal Foundation
SPF 18

PRINCIPAL DISPLAY PANEL - 30 ml Carton (204)
AMOREPACIFIC
TIME RESPONSE
Skin Renewal Foundation
SPF 18

PRINCIPAL DISPLAY PANEL - 30 ml Carton (206)
AMOREPACIFIC
TIME RESPONSE
Skin Renewal Foundation
SPF 18
AMOREPACIFICOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AMOREPACIFICOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AMOREPACIFICOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AMOREPACIFICOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AMOREPACIFICOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AMOREPACIFICOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||