Amlodipine Besylate
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use amlodipine besylate tablets safely and effectively. See full prescribing information for amlodipine besylate tablets. Amlodipine Besylate Tablets for oral administration Initial U.S. Approval: 1987INDICATIONS AND USAGE Hypertension (1.1) Coronary Artery Disease (1.2) Chronic Stable Angina Vasospastic Angina (Prinzmetal’s or Variant Angina) Angiographically Documented Coronary Artery Disease in patients without heart failure or an ejection fraction < 40% DOSAGE AND ADMINISTRATION Adult recommended starting dose: 5 mg once daily with maximum dose 10 mg once daily. (2.1) Small, fragile, or elderly patients, or patients with hepatic insufficiency may be started on 2.5 mg once daily. (2.1) Pediatric starting dose: 2.5 mg to 5 mg once daily. (2.2) Important Limitation: Doses in excess of 5 mg daily have not been studied in pediatric patients. (2.2)DOSAGE FORMS AND STRENGTHS 2.5 mg, 5 mg, and 10 mg Tablets (3) CONTRAINDICATIONS Known sensitivity to amlodipine (4) WARNINGS AND PRECAUTIONS Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. However, because of the gradual onset of action, acute hypotension is unlikely. (5.1) Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of amlodipine besylate, particularly in patients with severe obstructive coronary artery disease. (5.2) Titrate slowly when administering calcium channel blockers to patients with severe hepatic impairment. (5.4) Side EffectsTo report SUSPECTED ADVERSE REACTIONS, contact Wockhardt USA LLC. at 1-800-346-6854 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .USE IN SPECIFIC POPULATIONS Pregnancy: Use only if the potential benefit justifies the potential risk. (8.1) Nursing: Discontinue when administering amlodipine besylate. (8.3) Pediatric: Effect on patients less than 6 years old is not known. (8.4) Geriatric: Start dosing at the low end of the dose range, due to the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy. (8.5)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 AMLODIPINE BESYLATE INDICATIONS AND USAGE
- 2 AMLODIPINE BESYLATE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 AMLODIPINE BESYLATE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 AMLODIPINE BESYLATE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 AMLODIPINE BESYLATE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL AMLODIPINE BESYLATE TABLETS 2.5MG
- PRINCIPAL DISPLAY PANEL AMLODIPINE BESYLATE TABLETS 5MG
- PRINCIPAL DISPLAY PANEL AMLODIPINE BESYLATE TABLETS 10MG
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Hypertension
1.2 Coronary Artery Disease (CAD)
Chronic Stable AnginaVasospastic Angina (Prinzmetal's or Variant Angina)
Angiographically Documented CAD
2 DOSAGE AND ADMINISTRATION
2.1 Adults
[see Adverse Reactions (6)].
[see Clinical Studies (14.4)].
2.2 Children
[see Clinical Pharmacology (12.4), Clinical Studies (14.1)].3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension
5.2 Increased Angina or Myocardial Infarction
5.3 Beta-Blocker Withdrawal
5.4 Patients with Hepatic Failure
1/26 ADVERSE REACTIONS
6.1 Clinical Trials Experience
|
Adverse
Event
|
2.5 mg N=275 |
5 mg N=296 |
10 mg N=268 |
Placebo N=520 |
| Edema
|
1.8
|
3.0
|
10.8
|
0.6
|
| Dizziness
|
1.1
|
3.4
|
3.4
|
1.5
|
| Flushing
|
0.7
|
1.4
|
2.6
|
0.0
|
| Palpitation |
0.7
|
1.4
|
4.5
|
0.6
|
Other adverse experiences that were not clearly dose related but were reported with an incidence greater than 1.0% in placebo-controlled clinical trials include the following:
| |
Amlodipine Besylate (%) (N=1730) |
Placebo (%) (N=1250) |
| Headache |
7.3 |
7.8 |
| Fatigue |
4.5 |
2.8 |
| Nausea |
2.9 |
1.9 |
| Abdominal Pain |
1.6 |
0.3 |
| Somnolence |
1.4 |
0.6 |
For several adverse experiences that appear to be drug and dose related, there was a greater incidence in women than men associated with amlodipine treatment as shown in the following table:
| |
Amlodipine Besylate |
Placebo |
||
|
Adverse
Event
|
Male=% (N=1218) |
Female=% (N=512) |
Male=% (N=914) |
Female=% (N=336) |
| Edema |
5.6 |
14.6 |
1.4 |
5.1 |
| Flushing |
1.5 |
4.5 |
0.3 |
0.9 |
| Palpitations |
1.4 |
3.3 |
0.9 |
0.9 |
| Somnolence |
1.3 |
1.6 |
0.8 |
0.3 |
Cardiovascular:
Central and Peripheral Nervous System:
Gastrointestinal:1
General:1
Musculoskeletal System: 1
Psychiatric:1
Respiratory System: 1
Skin and Appendages: 11
Special Senses:
Urinary System:
Autonomic Nervous System:
Metabolic and Nutritional:
Hemopoietic:
1
[see Clinical Studies (14.4)]
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Data
In vitro7.2 Cimetidine
7.3 Grapefruit Juice
7.4 Magnesium and Aluminum Hydroxide Antacid
7.5 Sildenafil
7.6 Atorvastatin
7.7 Digoxin
7.8 Ethanol (Alcohol)
7.9 Warfarin
7.10 Drug/Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
222
2
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
[see Dosage and Administration (2.1)].10 OVERDOSAGE
2
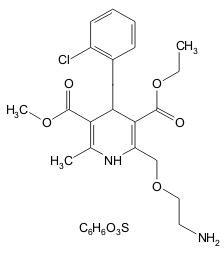
11 DESCRIPTION
202525663
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
in vitroin vitro
12.2 Pharmacodynamics
12.3 Pharmacokinetics and Metabolism
Ex vivo
12.4 Pediatric Patients
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
232332
3
14 CLINICAL STUDIES
14.1 Effects in Hypertension
Adult PatientsPediatric Patients
14.2 Effects in Chronic Stable Angina
14.3 Effects in Vasospastic Angina
14.4 Effects in Documented Coronary Artery Disease
Figure 1 - Kaplan-Meier Analysis of Composite Clinical Outcomes for Amlodipine Besylate versus Placebo
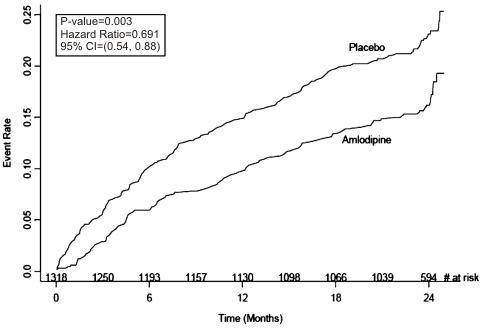
Figure 2 Effects on Primary Endpoint of Amlodipine Besylate versus Placebo across Sub-Groups

| Clinical Outcomes N (%) |
Amlodipine Besylate (N=663) |
Placebo (N=655) |
Risk Reduction (p-value) |
|---|---|---|---|
| Composite CV Endpoint | 110 (16.6) |
151
(23.1) |
31%
(0.003) |
| Hospitalization for Angina* | 51 (7.7) |
84 (12.8) |
42% (0.002) |
| Coronary Revascularization* | 78 (11.8) |
103 (15.7) |
27% (0.033) |
14.5 Studies in Patients with Heart Failure
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 2.5 mg Tablets
16.2 5 mg Tablets
16.3 10 mg Tablets
16.4 Storage
Store at 20° to 25°C (68° to 77°F) (USP Controlled Room Temperature) and dispense in tight, light-resistant containers (USP).
Manufactured by:
Wockhardt Limited
Mumbai, India.
Distributed by:
Wockhardt USA LLC.
20 Waterview Blvd.
Parsippany, NJ 07054
USA.
Distributed by:
MAJOR® PHARMACEUTIALS
Livonia, MI 48150
REFER TO PACKAGE LABEL FOR DISTRIBUTOR'S NDC NUMBER
Rev.131010
Amlodipine Besylate Tablets
amlodipine besylate tabletsamlodipine besylate tabletsamlodipine besylate tablets
What are amlodipine besylate tablets?
Amlodipine besylate tablets
High Blood Pressure (hypertension)
Amlodipine besylate tablets
Angina
Amlodipine besylate tablets
Who should not use amlodipine besylate tablets?
amlodipine besylate tabletsamlodipine besylate tablets
What should I tell my doctor before taking amlodipine besylate tablets?
- ever had heart disease
- ever had liver problems
- are pregnant, or plan to become pregnant. Your doctor will decide if amlodipine besylate tablets are the best treatment for you.
- are breast-feeding. Do not breast-feed while taking amlodipine besylate tablets. You can stop breast-feeding or take a different medicine.
- Take amlodipine besylate tablets once a day, with or without food. You can take amlodipine besylate tablets with most drinks, including grapefruit juice.
- It may be easier to take your dose if you do it at the same time every day, such as with breakfast or dinner, or at bedtime. Do not take more than one dose of amlodipine besylate tablets at a time.
- If you miss a dose, take it as soon as you remember. Do not take amlodipine besylate tablets if it has been more than 12 hours since you missed your last dose. Wait and take the next dose at your regular time.
- Other medicines: You can use nitroglycerin and amlodipine besylate tablets together. If you take nitroglycerin for angina, don't stop taking it while you are taking a mlodipine besylate tablets.
- While you are taking amlodipine besylate tablets, do not stop taking your other prescription medicines, including any other blood pressure medicines, without talking to your doctor.
- If you took too much amlodipine besylate tablets, call your doctor or Poison Control Center, or go to the nearest hospital emergency room right away.
- Do not breast-feed. It is not known if amlodipine besylate tablets will pass through your milk.
- Do not start any new prescription or non-prescription medicines or supplements, unless you check with your doctor first.
Amlodipine besylate tablets
- headache
- swelling of your legs or ankles
- tiredness, extreme sleepiness
- stomach pain, nausea
- dizziness
- flushing (hot or warm feeling in your face)
- arrhythmia (irregular heartbeat)
- heart palpitations (very fast heartbeat)
amlodipine besylate tablets
How do I store amlodipine besylate tablets?
amlodipine besylate tabletsamlodipine besylate tabletsamlodipine besylate tabletsamlodipine besylate tablets
General advice about amlodipine besylate tablets
amlodipine besylate tabletsamlodipine besylate tablets
amlodipine besylate tablets
Manufactured by:
Distributed by:
PRINCIPAL DISPLAY PANEL AMLODIPINE BESYLATE TABLETS 2.5MG

PRINCIPAL DISPLAY PANEL AMLODIPINE BESYLATE TABLETS 5MG

PRINCIPAL DISPLAY PANEL AMLODIPINE BESYLATE TABLETS 10MG

Amlodipine BesylateAmlodipine Besylate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Amlodipine BesylateAmlodipine Besylate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Amlodipine BesylateAmlodipine Besylate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||