AMIFOSTINE
Sun Pharmaceutical Industries Limited
Amifostine for Injection
FULL PRESCRIBING INFORMATION: CONTENTS*
- AMIFOSTINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- AMIFOSTINE INDICATIONS AND USAGE
- AMIFOSTINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- AMIFOSTINE ADVERSE REACTIONS
- OVERDOSAGE
- AMIFOSTINE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - vial label
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - showbox for 1 vial
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - showbox for 3 vials
FULL PRESCRIBING INFORMATION
AMIFOSTINE DESCRIPTION
Amifostine for injection is an organic thiophosphate cytoprotective agent known chemically as 2-[(3-aminopropyl) amino] ethanethiol dihydrogen phosphate (ester) and has the following structural formula:

Amifostine is a white crystalline powder, which is freely soluble in water. Its molecular formula is C5H15N2O3PS and it has a molecular weight of 214.22.
Amifostine for Injection is the trihydrate form of amifostine and is supplied as a sterile powder requiring reconstitution for intravenous infusion. Each single-use 10 mL vial contains 500 mg of amifostine on the anhydrous basis.
CLINICAL PHARMACOLOGY
Pharmacokinetics:
Clinical pharmacokinetic studies show that Amifostine is rapidly cleared from the plasma with a distribution half-life of <1 minute and an elimination half-life of approximately 8 minutes. Less than 10% of Amifostine remains in the plasma 6 minutes after drug administration. Amifostine is rapidly metabolized to an active free thiol metabolite. A disulfide metabolite is produced subsequently and is less active than the free thiol. After a 10-second bolus dose of 150 mg/m2 of Amifostine, renal excretion of the parent drug and its two metabolites was low during the hour following drug administration, averaging 0.69%, 2.64% and 2.22% of the administered dose for the parent, thiol and disulfide, respectively. Measurable levels of the free thiol metabolite have been found in bone marrow cells 5 to 8 minutes after intravenous infusion of Amifostine. Pretreatment with dexamethasone or metoclopramide has no effect on Amifostine pharmacokinetics.
Clinical Studies
Chemotherapy for Ovarian Cancer.
A randomized controlled trial compared six cycles of cyclophosphamide 1000 mg/m2, and cisplatin 100 mg/m2 with or without Amifostine pretreatment at 910 mg/m2, in two successive cohorts of 121 patients with advanced ovarian cancer. In both cohorts, after multiple cycles of chemotherapy, pretreatment with
Amifostine significantly reduced the cumulative renal toxicity associated with cisplatin as assessed by the proportion of patients who had ≥ 40% decrease in creatinine clearance from pretreatment values, protracted elevations in serum creatinine (>1.5 mg/dL), or severe hypomagnesemia. Subgroup analyses suggested that the effect of Amifostine was present in patients who had received nephrotoxic antibiotics, or who had preexisting diabetes or hypertension (and thus may have been at increased risk for significant nephrotoxicity), as well as in patients who lacked these risks. Selected analyses of the effects of Amifostine in reducing the cumulative renal toxicity of cisplatin in the randomized ovarian cancer study are provided in TABLES 1 and 2, below.
| Amifostine+CP | CP |
p-value (2-sided) |
|
| All Patients | 16/122 (13%) | 36/120 (30%) | 0.001 |
| First Cohort | 10/63 | 20/58 | 0.018 |
| Second Cohort | 6/59 | 16/62 | 0.026 |
|
NCI-CTC Grade: (mEq/L) |
0 >1.4 |
1 ≤1.4 to >1.1 |
2 ≤1.1 to >0.8 |
3 ≤0.8 to >0.5 |
4 ≤0.5 |
p-value
 |
|
All Patients Amifostine+CP CP |
92 73 |
13 18 |
3 7 |
0 5 |
0 1 |
0.001 |
|
First Cohort Amifostine+CP CP |
49 35 |
10 8 |
3 6 |
0 3 |
0 1 |
0.017 |
|
Second Cohort Amifostine+CP CP |
43 38 |
3 10 |
0 1 |
0 2 |
0 0 |
0.012 |
In the randomized ovarian cancer study, Amifostine had no detectable effect on the antitumor efficacy of cisplatin-cyclophosphamide chemotherapy. Objective response rates (including pathologically confirmed complete remission rates), time to progression, and survival duration were all similar in the Amifostine and control study groups. The table below summarizes the principal efficacy findings of the randomized ovarian cancer study.
| Amifostine +CP | CP | |
| Complete pathologic tumor response rate | 21.3% | 15.8% |
|
Time to progression (months) Median (± 95% CI) |
15.8 (13.2, 25.1) | 18.1 (12.5, 20.4) |
| Mean (± Std error) | 19.8 (±1.04) | 19.1 (±1.58) |
| Hazard ratio (95% Confidence Interval) |
.98 (.64, 1.4) | |
|
Survival (months) Median (± 95% CI) |
31.3 (28.3, 38.2) | 31.8 (26.3, 39.8) |
| Mean (± Std error) | 33.7 (±2.03) | 34.3 (±2.04) |
| Hazard ratio (95% Confidence Interval) |
.97 (.69, 1.32) | |
AMIFOSTINE INDICATIONS AND USAGE
Amifostine for injection is indicated to reduce the cumulative renal toxicity associated with repeated administration of cisplatin in patients with advanced ovarian cancer.WARNINGS
AMIFOSTINE CONTRAINDICATIONS
WARNINGS
DOSAGE AND ADMINISTRATIONADVERSE REACTIONSPRECAUTIONS
ADVERSE REACTIONSPRECAUTIONS
DOSAGE AND ADMINISTRATION
ADVERSE REACTIONS
PRECAUTIONS
General
DOSAGE AND ADMINISTRATIONDOSAGE AND ADMINISTRATION
- Any rash involving the lips or involving mucosa not known to be due to another etiology (e.g., radiation mucositis, herpes simplex, etc.)
- Erythematous, edematous, or bullous lesions on the palms of the hands or soles of the feet and/or other cutaneous reactions on the trunk (front, back, abdomen)
- Cutaneous reactions with associated fever or other constitutional symptoms
Drug Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Salmonella typhimuriumPregnancy
Nursing Mothers
Pediatric Use
Geriatric Use
AMIFOSTINE ADVERSE REACTIONS
Controlled Trials2TABLE 4
|
Phase III Ovarian Cancer Trial (WR-1) 910 mg/m2 _____________________________ Per Patient Per Infusion |
||
|
Nausea/Vomiting ≥Grade 3 All Grades |
36/122 (30%) 117/122 (96%) |
53/592 (9%) 520/592 (88%) |
|
Hypotension ≥Grade 3  All Grades |
10/122 (8%) 75/122 (61%) |
159/592 (27%) |
Clinical Trials and Pharmacovigilance Reports
WARNINGSPRECAUTIONS
WARNINGSPRECAUTIONS
OVERDOSAGE
22AMIFOSTINE DOSAGE AND ADMINISTRATION
2| Baseline Systolic Blood Pressure (mm Hg) | |||||
| <100 | 100 to 119 | 120 to 139 | 140 to 179 | ≥180 | |
| Decrease in systolic blood pressure during infusion of Amifostine for injection (mm Hg) | 20 | 25 | 30 | 40 | 50 |
2
3
HOW SUPPLIED
Amifostine for Injection USP is supplied as a sterile powder in 10 mL single-use vials (NDC 62756-581-40). Each single-use vial contains 500 mg of amifostine on the anhydrous basis. The vials are available packaged as follows:
1 pack - 1 vial per carton (NDC 62756-581-40).
3 pack - 3 vials per carton (NDC 62756-581-42).
Store the powder dosage form at Controlled Room Temperature 20°-25°C (68°-77°F) [See USP].
Distributed by:
Caraco Pharmaceutical Laboratories, Ltd.
1150 Elijah McCoy Drive, Detroit, MI 48202
Manufactured by:
Sun Pharmaceutical Ind. Ltd.
Acme Plaza, Andheri-Kurla Road,
Andheri (East), Mumbai -400 059, India.
PJPI0131A
ISS. 11/2009
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - vial label
NDC 62756-581-40
Amifostine for Injection USP
500 mg/vial
Sterile
Single use vials
For Intravenous Use
Rx only

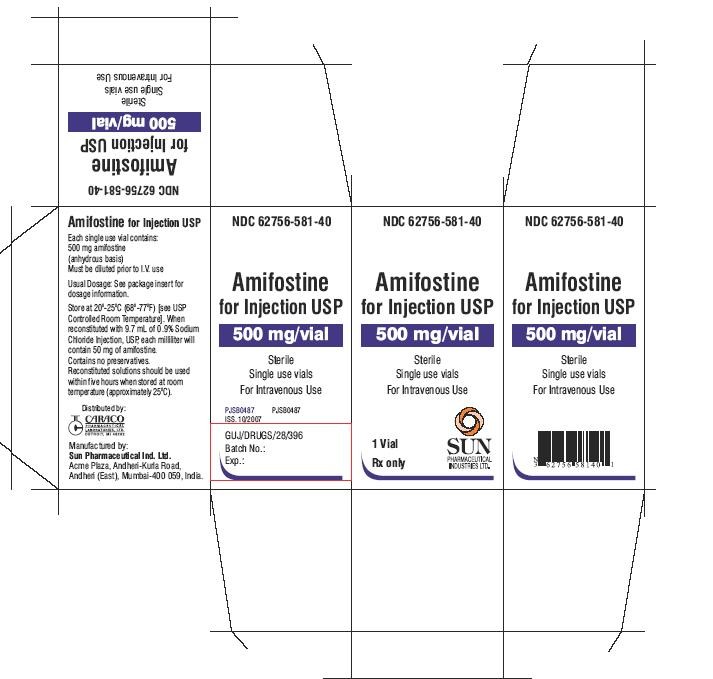
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - showbox for 1 vial
NDC 62756-581-40
Amifostine for Injection USP
500 mg/vial
Sterile
Single use vials
For Intravenous Use
1 Vial
Rx only
Sun Pharmaceutical Industries Ltd.
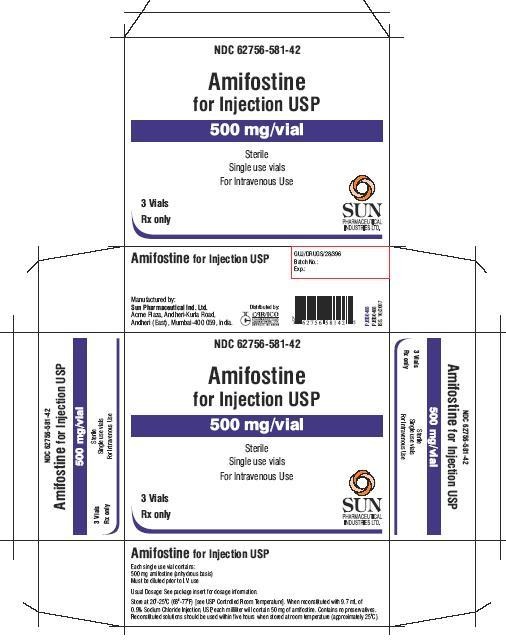
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - showbox for 3 vials
NDC 62756-581-42
Amifostine for Injection USP
500 mg/vial
Sterile
Single use vials
For Intravenous Use
3 Vials
Rx only
Sun Pharmaceutical Industries Ltd.
AMIFOSTINEAMIFOSTINE INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||