Alprazolam
FULL PRESCRIBING INFORMATION: CONTENTS*
- ALPRAZOLAM DESCRIPTION
- CLINICAL PHARMACOLOGY
- ALPRAZOLAM INDICATIONS AND USAGE
- ALPRAZOLAM CONTRAINDICATIONS
- WARNINGS
- Dependence and Withdrawal Reactions, Including Seizures
- Status Epilepticus and its Treatment
- Interdose Symptoms
- Risk of Dose Reduction
- Potent CYP 3A inhibitors
- Drugs demonstrated to be CYP 3A inhibitors on the basis of clinical studies involving alprazolam (caution and consideration of appropriate alprazolam dose reduction are recommended during coadministration with the following drugs)
- Other drugs possibly affecting alprazolam metabolism
- PRECAUTIONS
- ALPRAZOLAM ADVERSE REACTIONS
- Treatment-Emergent Adverse Events Reported in Placebo-Controlled Trials of Anxiety Disorders
- Treatment-Emergent Adverse Events Reported in Placebo-Controlled Trials of Panic Disorder
- Adverse Events Reported as Reasons for Discontinuation in Treatment of Panic Disorder in Placebo-Controlled Trials
- Post Introduction Reports
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- ALPRAZOLAM DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- ANIMAL STUDIES
- CLINICAL STUDIES
FULL PRESCRIBING INFORMATION
Rx Only
ALPRAZOLAM DESCRIPTION
Alprazolam Tablets USP contain alprazolam which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds.
The chemical name of alprazolam is 8-Chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-α] [1,4] benzodiazepine.
The structural formula is represented below:
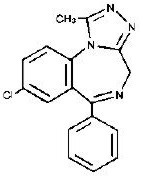
Alprazolam is a white crystalline powder, which is soluble in methanol or ethanol but which has no appreciable solubility in water at physiological pH.
Each Alprazolam Tablet USP, for oral administration, contains 0.25, 0.5, 1 or 2 mg of alprazolam.
Alprazolam Tablets USP, 2 mg, are multi-scored and may be divided as shown below:

Inactive Ingredients: Each tablet contains the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, mannitol and microcrystalline cellulose. In addition, the 0.5 mg tablet contains FD&C Yellow No. 6 Aluminum Lake and the 1 mg tablet contains FD&C Blue No. 2 Aluminum Lake.
CLINICAL PHARMACOLOGY
CNS agents of the 1,4 benzodiazepine class presumably exert their effects by binding at stereo specific receptors at several sites within the central nervous system. Their exact mechanism of action is unknown. Clinically, all benzodiazepines cause a dose-related central nervous system depressant activity varying from mild impairment of task performance to hypnosis.
Following oral administration, alprazolam is readily absorbed. Peak concentrations in the plasma occur in one to two hours following administration. Plasma levels are proportionate to the dose given; over the dose range of 0.5 to 3.0 mg, peak levels of 8.0 to 37 ng/mL were observed. Using a specific assay methodology, the mean plasma elimination half-life of alprazolam has been found to be about 11.2 hours (range: 6.3-26.9 hours) in healthy adults.
The predominant metabolites are (alpha)-hydroxy-alprazolam and a benzophenone derived from alprazolam. The biological activity of (alpha)-hydroxy-alprazolam is approximately one-half that of alprazolam. The benzophenone metabolite is essentially inactive. Plasma levels of these metabolites are extremely low, thus precluding precise pharmacokinetic description. However, their half-lives appear to be of the same order of magnitude as that of alprazolam. Alprazolam and its metabolites are excreted primarily in the urine.
The ability of alprazolam to induce human hepatic enzyme systems has not yet been determined. However, this is not a property of benzodiazepines in general. Further, alprazolam did not affect the prothrombin or plasma warfarin levels in male volunteers administered sodium warfarin orally.
In vitro, alprazolam is bound (80 percent) to human serum protein.
Changes in the absorption, distribution, metabolism and excretion of benzodiazepines have been reported in a variety of disease states including alcoholism, impaired hepatic function and impaired renal function. Changes have also been demonstrated in geriatric patients. A mean half-life of alprazolam of 16.3 hours has been observed in healthy elderly subjects (range: 9.0-26.9 hours, n=16) compared to 11.0 hours (range: 6.3-15.8 hours, n=16) in healthy adult subjects. In patients with alcoholic liver disease the half-life of alprazolam ranged between 5.8 and 65.3 hours (mean: 19.7 hours, n=17) as compared to between 6.3 and 26.9 hours (mean=11.4 hours, n=17) in healthy subjects. In an obese group of subjects the half-life of alprazolam ranged between 9.9 and 40.4 hours (mean=21.8 hours, n=12) as compared to between 6.3 and 15.8 hours (mean=10.6 hours, n=12) in healthy subjects.
Because of its similarity to other benzodiazepines, it is assumed that alprazolam undergoes transplacental passage and that it is excreted in human milk.
ALPRAZOLAM INDICATIONS AND USAGE
Alprazolam Tablets USP are indicated for the management of anxiety disorder (a condition corresponding most closely to the APA Diagnostic and Statistical Manual [DSM-III-R] diagnosis of generalized anxiety disorder) or the short-term relief of symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
Generalized anxiety disorder is characterized by unrealistic or excessive anxiety and worry (apprehensive expectation) about two or more life circumstances, for a period of six months or longer, during which the person has been bothered more days than not by these concerns. At least 6 of the following 18 symptoms are often present in these patients: Motor Tension (trembling, twitching, or feeling shaky; muscle tension, aches, or soreness; restlessness; easy fatigability); Autonomic Hyperactivity (shortness of breath or smothering sensations; palpitations or accelerated heart rate; sweating, or cold clammy hands; dry mouth; dizziness or light-headedness; nausea, diarrhea, or other abdominal distress; flushes or chills; frequent urination; trouble swallowing or 'lump in throat'); Vigilance and Scanning (feeling keyed up or on edge; exaggerated startle response; difficulty concentrating or `mind going blank' because of anxiety; trouble falling or staying asleep; irritability). These symptoms must not be secondary to another psychiatric disorder or caused by some organic factor.
Anxiety associated with depression is responsive to Alprazolam Tablets USP.
Alprazolam Tablets USP is also indicated for the treatment of panic disorder, with or without agoraphobia.
Studies supporting this claim were conducted in patients whose diagnoses corresponded closely to the DSM-III-R criteria for panic disorder (see CLINICALSTUDIES).
Panic disorder is an illness characterized by recurrent panic attacks. The panic attacks, at least initially, are unexpected. Later in the course of this disturbance certain situations, eg, driving a car or being in a crowded place, may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterized by at least four of the following symptoms: dyspnea or smothering sensations; dizziness, unsteady feelings, or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalization or derealization; paresthesias; hot flashes or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. At least some of the panic attack symptoms must develop suddenly, and the panic attack symptoms must not be attributable to some known organic factors. Panic disorder is frequently associated with some symptoms of agoraphobia.
Demonstrations of the effectiveness of Alprazolam Tablets USP by systematic clinical study are limited to four months duration for anxiety disorder and four to ten weeks duration for panic disorder; however, patients with panic disorder have been treated on an open basis for up to eight months without apparent loss of benefit. The physician should periodically reassess the usefulness of the drug for the individual patient.
ALPRAZOLAM CONTRAINDICATIONS
Alprazolam Tablets USP are contraindicated in patients with known sensitivity to this drug or other benzodiazepines. Alprazolam Tablets USP may be used in patients with open angle glaucoma who are receiving appropriate therapy, but is contraindicated in patients with acute narrow angle glaucoma.
Alprazolam Tablets USP is contraindicated with ketoconazole and itraconazole, since these medications significantly impair the oxidative metabolism mediated by cytochrome P450 3A (CYP 3A) (see WARNINGS and PRECAUTIONS: Drug Interactions).
WARNINGS
Dependence and Withdrawal Reactions, Including Seizures
Certain adverse clinical events, some life-threatening, are a direct consequence of physical dependence to Alprazolam Tablets USP. These include a spectrum of withdrawal symptoms; the most important is seizure (see DRUG ABUSE AND DEPENDENCE). Even after relatively short-term use at the doses recommended for the treatment of transient anxiety and anxiety disorder (ie, 0.75 to 4.0 mg per day), there is some risk of dependence. Spontaneous reporting system data suggest that the risk of dependence and its severity appear to be greater in patients treated with doses greater than 4 mg/day and for long periods (more than 12 weeks). However, in a controlled postmarketing discontinuation study of panic disorder patients, the duration of treatment (three months compared to six months) had no effect on the ability of patients to taper to zero dose. In contrast, patients treated with doses of Alprazolam Tablets USP greater than 4 mg/day had more difficulty tapering to zero dose than those treated with less than 4 mg/day.
The importance of dose and the risks of Alprazolam Tablets USP as a treatment for panic disorder
Because the management of panic disorder often requires the use of average daily doses of Alprazolam Tablets USP above 4 mg, the risk of dependence among panic disorder patients may be higher than that among those treated for less severe anxiety. Experience in randomized placebo-controlled discontinuation studies of patients with panic disorder showed a high rate of rebound and withdrawal symptoms in patients treated with Alprazolam Tablets USP compared to placebo treated patients.
Relapse or return of illness was defined as a return of symptoms characteristic of panic disorder (primarily panic attacks) to levels approximately equal to those seen at baseline before active treatment was initiated. Rebound refers to a return of symptoms of panic disorder to a level substantially greater in frequency, or more severe in intensity than seen at baseline. Withdrawal symptoms were identified as those which were generally not characteristic of panic disorder and which occurred for the first time more frequently during discontinuation than at baseline.
In a controlled clinical trial in which 63 patients were randomized to Alprazolam Tablets USP and where withdrawal symptoms were specifically sought, the following were identified as symptoms of withdrawal: heightened sensory perception, impaired concentration, dysosmia, clouded sensorium, paresthesias, muscle cramps, muscle twitch, diarrhea, blurred vision, appetite decrease and weight loss. Other symptoms, such as anxiety and insomnia, were frequently seen during discontinuation, but it could not be determined if they were due to return of illness, rebound or withdrawal.
In a larger database comprised of both controlled and uncontrolled studies in which 641 patients received Alprazolam Tablets USP, discontinuation-emergent symptoms which occurred at a rate of over 5% in patients treated with Alprazolam Tablets USP and at a greater rate than the placebo treated group were as follows:
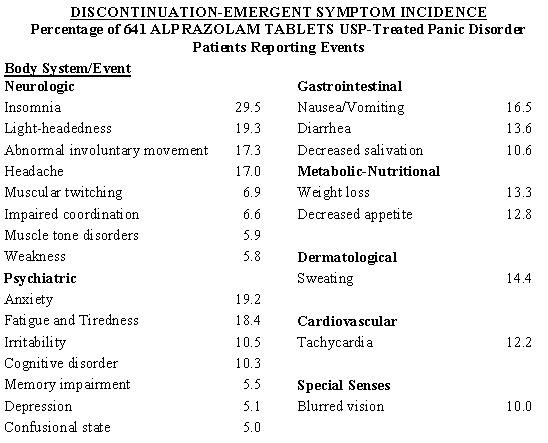
From the studies cited, it has not been determined whether these symptoms are clearly related to the dose and duration of therapy with Alprazolam Tablets USP in patients with panic disorder.
In two controlled trials of six to eight weeks duration where the ability of patients to discontinue medication was measured, 71%-93% of Alprazolam Tablets USP treated patients tapered completely off therapy compared to 89%-96% of placebo treated patients. In a controlled postmarketing discontinuation study of panic disorder patients, the duration of treatment (three months compared to six months) had no effect on the ability of patients to taper to zero dose.
Seizures attributable to Alprazolam Tablets USP were seen after drug discontinuance or dose reduction in 8 of 1980 patients with panic disorder or in patients participating in clinical trials where doses of Alprazolam Tablets USP greater than 4 mg/day for over 3 months were permitted. Five of these cases clearly occurred during abrupt dose reduction, or discontinuation from daily doses of 2 to 10 mg. Three cases occurred in situations where there was not a clear relationship to abrupt dose reduction or discontinuation. In one instance, seizure occurred after discontinuation from a single dose of 1 mg after tapering at a rate of 1 mg every three days from 6 mg daily. In two other instances, the relationship to taper is indeterminate; in both of these cases the patients had been receiving doses of 3 mg daily prior to seizure. The duration of use in the above 8 cases ranged from 4 to 22 weeks. There have been occasional voluntary reports of patients developing seizures while apparently tapering gradually from Alprazolam Tablets USP. The risk of seizure seems to be greatest 24-72 hours after discontinuation (see DOSAGE AND ADMINISTRATION for recommended tapering and discontinuation schedule).
Status Epilepticus and its Treatment
The medical event voluntary reporting system shows that withdrawal seizures have been reported in association with the discontinuation of Alprazolam Tablets USP. In most cases, only a single seizure was reported; however, multiple seizures and status epilepticus were reported as well. Ordinarily, the treatment of status epilepticus of any etiology involves use of intravenous benzodiazepines plus phenytoin or barbiturates, maintenance of a patent airway and adequate hydration. For additional details regarding therapy, consultation with an appropriate specialist may be considered.
Interdose Symptoms
Early morning anxiety and emergence of anxiety symptoms between doses of Alprazolam Tablets USP have been reported in patients with panic disorder taking prescribed maintenance doses of Alprazolam Tablets USP. These symptoms may reflect the development of tolerance or a time interval between doses which is longer than the duration of clinical action of the administered dose. In either case, it is presumed that the prescribed dose is not sufficient to maintain plasma levels above those needed to prevent relapse, rebound or withdrawal symptoms over the entire course of the interdosing interval. In these situations, it is recommended that the same total daily dose be given divided as more frequent administrations (see DOSAGE AND ADMINISTRATION).
Risk of Dose Reduction
Withdrawal reactions may occur when dosage reduction occurs for any reason. This includes purposeful tapering, but also inadvertent reduction of dose (eg, the patient forgets, the patient is admitted to a hospital, etc.). Therefore, the dosage of Alprazolam Tablets USP should be reduced or discontinued gradually (see DOSAGE AND ADMINISTRATION).
Alprazolam Tablets USP are not of value in the treatment of psychotic patients and should not be employed in lieu of appropriate treatment for psychosis. Because of its CNS depressant effects, patients receiving Alprazolam Tablets USP should be cautioned against engaging in hazardous occupations or activities requiring complete mental alertness such as operating machinery or driving a motor vehicle. For the same reason, patients should be cautioned about the simultaneous ingestion of alcohol and other CNS depressant drugs during treatment with Alprazolam Tablets USP.
Benzodiazepines can potentially cause fetal harm when administered to pregnant women. If Alprazolam Tablets USP is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Because of experience with other members of the benzodiazepine class, Alprazolam Tablets USP is assumed to be capable of causing an increased risk of congenital abnormalities when administered to a pregnant woman during the first trimester. Because use of these drugs is rarely a matter of urgency, their use during the first trimester should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physicians about the desirability of discontinuing the drug.
Alprazolam interaction with drugs that inhibit metabolism via cytochrome P450 3A:
The initial step in alprazolam metabolism is hydroxylation catalyzed by cytochrome P450 3A (CYP 3A). Drugs that inhibit this metabolic pathway may have a profound effect on the clearance of alprazolam. Consequently, alprazolam should be avoided in patients receiving very potent inhibitors of CYP 3A. With drugs inhibiting CYP 3A to a lesser but still significant degree, alprazolam should be used only with caution and consideration of appropriate dosage reduction. For some drugs, an interaction with alprazolam has been quantified with clinical data; for other drugs, interactions are predicted from in vitro data and/or experience with similar drugs in the same pharmacologic class.
The following are examples of drugs known to inhibit the metabolism of alprazolam and/or related benzodiazepines, presumably through inhibition of CYP 3A.
Potent CYP 3A inhibitors
Azole antifungal agents --Although in vivo interaction data with alprazolam are not available, ketoconazole and itraconazole are potent CYP 3A inhibitors and the coadministration of alprazolam with them is not recommended. Other azole-type antifungal agents should also be considered potent CYP 3A inhibitors and the coadministration of alprazolam with them is not recommended (see CONTRAINDICATIONS).
Drugs demonstrated to be CYP 3A inhibitors on the basis of clinical studies involving alprazolam (caution and consideration of appropriate alprazolam dose reduction are recommended during coadministration with the following drugs)
Nefazodone --Coadministration of nefazodone increased alprazolam concentration two-fold.
Fluvoxamine --Coadministration of fluvoxamine approximately doubled the maximum plasma concentration of alprazolam, decreased clearance by 49%, increased half-life by 71%, and decreased measured psychomotor performance.
Cimetidine --Coadministration of cimetidine increased the maximum plasma concentration of alprazolam by 86%, decreased clearance by 42%, and increased half-life by 16%.
Other drugs possibly affecting alprazolam metabolism
Other drugs possibly affecting alprazolam metabolism by inhibition of CYP 3A are discussed in the PRECAUTIONS section (see PRECAUTIONS : Drug Interactions).
PRECAUTIONS
General
If Alprazolam Tablets USP are to be combined with other psychotropic agents or anticonvulsant drugs, careful consideration should be given to the pharmacology of the agents to be employed, particularly with compounds which might potentiate the action of benzodiazepines (see DRUG INTERACTIONS).
As with other psychotropic medications, the usual precautions with respect to administration of the drug and size of the prescription are indicated for severely depressed patients or those in whom there is reason to expect concealed suicidal ideation or plans.
It is recommended that the dosage be limited to the smallest effective dose to preclude the development of ataxia or oversedation which may be a particular problem in elderly or debilitated patients. (See DOSAGE AND ADMINISTRATION.) The usual precautions in treating patients with impaired renal, hepatic or pulmonary function should be observed. There have been rare reports of death in patients with severe pulmonary disease shortly after the initiation of treatment with Alprazolam Tablets USP. A decreased systemic alprazolam elimination rate (eg, increased plasma half-life) has been observed in both alcoholic liver disease patients and obese patients receiving Alprazolam Tablets USP (see CLINICAL PHARMACOLOGY).
Episodes of hypomania and mania have been reported in association with the use of Alprazolam Tablets USP in patients with depression.
Alprazolam has a weak uricosuric effect. Although other medications with weak uricosuric effect have been reported to cause acute renal failure, there have been no reported instances of acute renal failure attributable to therapy with Alprazolam Tablets USP.
Information for Patients
To assure safe and effective use of benzodiazepines, all patients prescribed Alprazolam Tablets USP should be provided with the following guidance. In addition, panic disorder patients, for whom doses greater than 4 mg/day are typically prescribed, should be advised about the risks associated with the use of higher doses.
1. Inform your physician about any alcohol consumption and medicine you are taking now, including medication you may buy without a prescription. Alcohol should generally not be used during treatment with benzodiazepines.
2. Not recommended for use in pregnancy. Therefore, inform your physician if you are pregnant, if you are planning to have a child, or if you become pregnant while you are taking this medication.
3. Inform your physician if you are nursing.
4. Until you experience how this medication affects you, do not drive a car or operate potentially dangerous machinery, etc.
5. Do not increase the dose even if you think the medication "does not work anymore" without consulting your physician. Benzodiazepines, even when used as recommended, may produce emotional and/or physical dependence.
6. Do not stop taking this medication abruptly or decrease the dose without consulting your physician, since withdrawal symptoms can occur.
Additional advice for panic disorder patients
The use of Alprazolam Tablets USP at doses greater than 4 mg/day, often necessary to treat panic disorder, is accompanied by risks that you need to carefully consider. When used at doses greater than 4 mg/day, which may or may not be required for your treatment, Alprazolam Tablets USP has the potential to cause severe emotional and physical dependence in some patients and these patients may find it exceedingly difficult to terminate treatment. In two controlled trials of six to eight weeks duration where the ability of patients to discontinue medication was measured, 7 to 29% of patients treated with Alprazolam Tablets USP did not completely taper off therapy. In a controlled postmarketing discontinuation study of panic disorder patients, the patients treated with doses of Alprazolam Tablets USP greater than 4 mg/day had more difficulty tapering to zero dose than patients treated with less than 4 mg/day. In all cases, it is important that your physician help you discontinue this medication in a careful and safe manner to avoid overly extended use of Alprazolam Tablets USP.
In addition, the extended use at doses greater than 4 mg/day appears to increase the incidence and severity of withdrawal reactions when Alprazolam Tablets USP is discontinued. These are generally minor but seizure can occur, especially if you reduce the dose too rapidly or discontinue the medication abruptly. Seizure can be life-threatening.
Laboratory Tests
Laboratory tests are not ordinarily required in otherwise healthy patients.
Drug Interactions
The benzodiazepines, including alprazolam, produce additive CNS depressant effects when co-administered with other psychotropic medications, anticonvulsants, antihistaminics, ethanol and other drugs which themselves produce CNS depression. The steady state plasma concentrations of imipramine and desipramine have been reported to be increased an average of 31% and 20%, respectively, by the concomitant administration of Alprazolam Tablets USP in doses up to 4 mg/day. The clinical significance of these changes is unknown.
Drugs that inhibit alprazolam metabolism via cytochrome P450 3A:
The initial step in alprazolam metabolism is hydroxylation catalyzed by cytochrome P450 3A (CYP 3A). Drugs which inhibit this metabolic pathway may have a profound effect on the clearance of alprazolam (see CONTRAINDICATIONS and WARNINGS for additional drugs of this type).
Drugs demonstrated to be CYP 3A inhibitors of possible clinical significance on the basis of clinical studies involving alprazolam (caution is recommended during coadministration with alprazolam)
Fluoxetine--Coadministration of fluoxetine with alprazolam increased the maximum plasma concentration of alprazolam by 46%, decreased clearance by 21%, increased half-life by 17%, and decreased measured psychomotor performance.
Propoxyphene--Coadministration of propoxyphene decreased the maximum plasma concentration of alprazolam by 6%, decreased clearance by 38%, and increased half-life by 58%.
Oral Contraceptives--Coadministration of oral contraceptives increased the maximum plasma concentration of alprazolam by 18%, decreased clearance by 22%, and increased half-life by 29%.
Drugs and other substances demonstrated to be CYP 3A inhibitors on the basis of clinical studies involving benzodiazepines metabolized similarly to alprazolam or on the basis of in vitro studies with alprazolam or other benzodiazepines (caution is recomme
Available data from clinical studies of benzodiazepines other than alprazolam suggest a possible drug interaction with alprazolam for the following: diltiazem, isoniazid, macrolide antibiotics such as erythromycin and clarithromycin, and grapefruit juice. Data from in vitro studies of alprazolam suggest a possible drug interaction with alprazolam for the following: sertraline and paroxetine. Data from in vitro studies of benzodiazepines other than alprazolam suggest a possible drug interaction for the following: ergotamine, cyclosporine, amiodarone, nicradipine, and nifedipine. Caution is recommended during the coadministration of any of these with alprazolam (see WARNINGS).
Drug/Laboratory Test Interactions
Although interactions between benzodiazepines and commonly employed clinical laboratory tests have occasionally been reported, there is no consistent pattern for a specific drug or specific test.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of carcinogenic potential was observed during 2-year bioassay studies of alprazolam in rats at doses up to 30 mg/kg/day (150 times the maximum recommended daily human dose of 10 mg/day) and in mice at doses up to 10 mg/kg/day (50 times the maximum recommended daily human dose).
Alprazolam was not mutagenic in the rat micronucleus test at doses up to 100 mg/kg, which is 500 times the maximum recommended daily human dose of 10 mg/day. Alprazolam also was not mutagenic in vitro in the DNA Damage/Alkaline Elution Assay or the Ames Assay.
Alprazolam produced no impairment of fertility in rats at doses up to 5 mg/kg/day, which is 25 times the maximum recommended daily human dose of 10 mg/day.
Pregnancy
Teratogenic Effects: Pregnancy Category D: (See WARNINGS Section).
Nonteratogenic Effects: It should be considered that the child born of a mother who is receiving benzodiazepines may be at some risk for withdrawal symptoms from the drug during the postnatal period. Also, neonatal flaccidity and respiratory problems have been reported in children born of mothers who have been receiving benzodiazepines.
Labor and Delivery
Alprazolam Tablets USP has no established use in labor or delivery.
Nursing Mothers
Benzodiazepines are known to be excreted in human milk. It should be assumed that alprazolam is as well. Chronic administration of diazepam to nursing mothers has been reported to cause their infants to become lethargic and to lose weight. As a general rule, nursing should not be undertaken by mothers who must use Alprazolam Tablets USP.
Pediatric Use
Safety and effectiveness of Alprazolam Tablets USP in individuals below 18 years of age have not been established.
Geriatric Use
The elderly may be more sensitive to the effects of benzodiazepines. They exhibit higher plasma alprazolam concentrations due to reduced clearance of the drug as compared with a younger population receiving the same doses. The smallest effective dose of Alprazolam Tablets USP should be used in the elderly to preclude the development of ataxia and oversedation (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
ALPRAZOLAM ADVERSE REACTIONS
Side effects to Alprazolam Tablets USP, if they occur, are generally observed at the beginning of therapy and usually disappear upon continued medication. In the usual patient, the most frequent side effects are likely to be an extension of the pharmacological activity of alprazolam, eg, drowsiness or light-headedness.
The data cited in the two tables below are estimates of untoward clinical event incidence among patients who participated under the following clinical conditions: relatively short duration (ie, four weeks) placebo-controlled clinical studies with dosages up to 4 mg/day of Alprazolam Tablets USP (for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety) and short-term (up to ten weeks) placebo-controlled clinical studies with dosages up to 10 mg/day of Alprazolam Tablets USP in patients with panic disorder, with or without agoraphobia.
These data cannot be used to predict precisely the incidence of untoward events in the course of usual medical practice where patient characteristics, and other factors often differ from those in clinical trials. These figures cannot be compared with those obtained from other clinical studies involving related drug products and placebo as each group of drug trials are conducted under a different set of conditions.
Comparison of the cited figures, however, can provide the prescriber with some basis for estimating the relative contributions of drug and non-drug factors to the untoward event incidence in the population studied. Even this use must be approached cautiously, as a drug may relieve a symptom in one patient but induce it in others. (For example, an anxiolytic drug may relieve dry mouth [a symptom of anxiety] in some subjects but induce it [an untoward event] in others.)
Additionally, for anxiety disorders the cited figures can provide the prescriber with an indication as to the frequency with which physician intervention (eg, increased surveillance, decreased dosage or discontinuation of drug therapy) may be necessary because of the untoward clinical event.
Treatment-Emergent Adverse Events Reported in Placebo-Controlled Trials of Anxiety Disorders
| ANXIETY DISORDERS | |||
|---|---|---|---|
| Treatment-Emergent Symptom Incidence | Incidence of Intervention Because of Symptom | ||
| Alprazolam Tablets USP | PLACEBO | Alprazolam Tablets USP | |
| Number of Patients % of Patients Reporting: | 565 | 505 | 565 |
| Central Nervous System | |||
| Drowsiness | 41.0 | 21.6 | 15.1 |
| Light-headedness | 20.8 | 19.3 | 1.2 |
| Depression | 13.9 | 18.1 | 2.4 |
| Headache | 12.9 | 19.6 | 1.1 |
| Confusion | 9.9 | 10.0 | 0.9 |
| Insomnia | 8.9 | 18.4 | 1.3 |
| Nervousness | 4.1 | 10.3 | 1.1 |
| Syncope | 3.1 | 4.0 |  |
| Dizziness | 1.8 | 0.8 | 2.5 |
| Akathisia | 1.6 | 1.2 |  |
| Tiredness/Sleepiness |  |  | 1.8 |
| Gastrointestinal | |||
| Dry Mouth | 14.7 | 13.3 | 0.7 |
| Constipation | 10.4 | 11.4 | 0.9 |
| Diarrhea | 10.1 | 10.3 | 1.2 |
| Nausea/Vomiting | 9.6 | 12.8 | 1.7 |
| Increased Salivation | 4.2 | 2.4 |  |
| Cardiovascular | |||
| Tachycardia/Palpitations | 7.7 | 15.6 | 0.4 |
| Hypotension | 4.7 | 2.2 |  |
| Sensory | |||
| Blurred Vision | 6.2 | 6.2 | 0.4 |
| Musculoskeletal | |||
| Rigidity | 4.2 | 5.3 |  |
| Tremor | 4.0 | 8.8 | 0.4 |
| Cutaneous | |||
| Dermatitis/Allergy | 3.8 | 3.1 | 0.6 |
| Other | |||
| Nasal Congestion | 7.3 | 9.3 |  |
| Weight Gain | 2.7 | 2.7 |  |
| Weight Loss | 2.3 | 3.0 |  |
In addition to the relatively common (ie, greater than 1%) untoward events enumerated in the table above, the following adverse events have been reported in association with the use of benzodiazepines: dystonia, irritability, concentration difficulties, anorexia, transient amnesia or memory impairment, loss of coordination, fatigue, seizures, sedation, slurred speech, jaundice, musculoskeletal weakness, pruritus, diplopia, dysarthria, changes in libido, menstrual irregularities, incontinence and urinary retention.
Treatment-Emergent Adverse Events Reported in Placebo-Controlled Trials of Panic Disorder
| PANIC DISORDER | ||
|---|---|---|
| Treatment-Emergent Symptom Incidence | ||
| Alprazolam Tablets USP | PLACEBO | |
| Number of Patients % of Patients Reporting: | 1388 | 1231 |
| Central Nervous System | ||
| Drowsiness | 76.8 | 42.7 |
| Fatigue and Tiredness | 48.6 | 42.3 |
| Impaired Coordination | 40.1 | 17.9 |
| Irritability | 33.1 | 30.1 |
| Memory Impairment | 33.1 | 22.1 |
| Light-headedness/Dizziness | 29.8 | 36.9 |
| Insomnia | 29.4 | 41.8 |
| Headache | 29.2 | 35.6 |
| Cognitive Disorder | 28.8 | 20.5 |
| Dysarthria | 23.3 | 6.3 |
| Anxiety | 16.6 | 24.9 |
| Abnormal Involuntary Movement | 14.8 | 21.0 |
| Decreased Libido | 14.4 | 8.0 |
| Depression | 13.8 | 14.0 |
| Confusional State | 10.4 | 8.2 |
| Muscular Twitching | 7.9 | 11.8 |
| Increased Libido | 7.7 | 4.1 |
| Change in Libido (Not Specified) | 7.1 | 5.6 |
| Weakness | 7.1 | 8.4 |
| Muscle Tone Disorders | 6.3 | 7.5 |
| Syncope | 3.8 | 4.8 |
| Akathisia | 3.0 | 4.3 |
| Agitation | 2.9 | 2.6 |
| Disinhibition | 2.7 | 1.5 |
| Paresthesia | 2.4 | 3.2 |
| Talkativeness | 2.2 | 1.0 |
| Vasomotor Disturbances | 2.0 | 2.6 |
| Derealization | 1.9 | 1.2 |
| Dream Abnormalities | 1.8 | 1.5 |
| Fear | 1.4 | 1.0 |
| Feeling Warm | 1.3 | 0.5 |
| Gastrointestinal | ||
| Decreased Salivation | 32.8 | 34.2 |
| Constipation | 26.2 | 15.4 |
| Nausea/Vomiting | 22.0 | 31.8 |
| Diarrhea | 20.6 | 22.8 |
| Abdominal Distress | 18.3 | 21.5 |
| Increased Salivation | 5.6 | 4.4 |
| Cardio-Respiratory | ||
| Nasal Congestion | 17.4 | 16.5 |
| Tachycardia | 15.4 | 26.8 |
| Chest Pain | 10.6 | 18.1 |
| Hyperventilation | 9.7 | 14.5 |
| Upper Respiratory Infection | 4.3 | 3.7 |
| Sensory | ||
| Blurred Vision | 21.0 | 21.4 |
| Tinnitus | 6.6 | 10.4 |
| Musculoskeletal | ||
| Muscular Cramps | 2.4 | 2.4 |
| Muscle Stiffness | 2.2 | 3.3 |
| Cutaneous | ||
| Sweating | 15.1 | 23.5 |
| Rash | 10.8 | 8.1 |
| Other | ||
| Increased Appetite | 32.7 | 22.8 |
| Decreased Appetite | 27.8 | 24.1 |
| Weight Gain | 27.2 | 17.9 |
| Weight Loss | 22.6 | 16.5 |
| Micturition Difficulties | 12.2 | 8.6 |
| Menstrual Disorders | 10.4 | 8.7 |
| Sexual Dysfunction | 7.4 | 3.7 |
| Edema | 4.9 | 5.6 |
| Incontinence | 1.5 | 0.6 |
| Infection | 1.3 | 1.7 |
In addition to the relatively common (ie, greater than 1%) untoward events enumerated in the table above, the following adverse events have been reported in association with the use of Alprazolam Tablets USP: seizures, hallucinations, depersonalization, taste alterations, diplopia, elevated bilirubin, elevated hepatic enzymes, and jaundice.
There have also been reports of withdrawal seizures upon rapid decrease or abrupt discontinuation of Alprazolam Tablets USP (see WARNINGS).
Adverse Events Reported as Reasons for Discontinuation in Treatment of Panic Disorder in Placebo-Controlled Trials
To discontinue treatment in patients taking Alprazolam Tablets USP, the dosage should be reduced slowly in keeping with good medical practice. It is suggested that the daily dosage of Alprazolam Tablets USP be decreased by no more than 0.5 mg every three days (see DOSAGE AND ADMINISTRATION). Some patients may benefit from an even slower dosage reduction. In a controlled postmarketing discontinuation study of panic disorder patients which compared this recommended taper schedule with a slower taper schedule, no difference was observed between the groups in the proportion of patients who tapered to zero dose; however, the slower schedule was associated with a reduction in symptoms associated with a withdrawal syndrome.
Panic disorder has been associated with primary and secondary major depressive disorders and increased reports of suicide among untreated patients. Therefore, the same precaution must be exercised when using doses of Alprazolam Tablets USP greater than 4 mg/day in treating patients with panic disorders as is exercised with the use of any psychotropic drug in treating depressed patients or those in whom there is reason to expect concealed suicidal ideation or plans.
As with all benzodiazepines, paradoxical reactions such as stimulation, increased muscle spasticity, sleep disturbances, hallucinations and other adverse behavioral effects such as agitation, rage, irritability, and aggressive or hostile behavior have been reported rarely. In many of the spontaneous case reports of adverse behavioral effects, patients were receiving other CNS drugs concomitantly and/or were described as having underlying psychiatric conditions. Should any of the above events occur, alprazolam should be discontinued. Isolated published reports involving small numbers of patients have suggested that patients who have borderline personality disorder, a prior history of violent or aggressive behavior, or alcohol or substance abuse may be at risk for such events. Instances of irritability, hostility, and intrusive thoughts have been reported during discontinuation of alprazolam in patients with post-traumatic stress disorder.
Laboratory analyses were performed on patients participating in the clinical program for Alprazolam Tablets USP. The following incidences of abnormalities shown below were observed in patients receiving Alprazolam Tablets USP and in patients in the corresponding placebo group. Few of these abnormalities were considered to be of physiological significance.

When treatment with Alprazolam Tablets USP is protracted, periodic blood counts, urinalysis and blood chemistry analyses are advisable.
Minor changes in EEG patterns, usually low-voltage fast activity have been observed in patients during therapy with Alprazolam Tablets USP and are of no known significance.
Post Introduction Reports
Various adverse drug reactions have been reported in association with the use of Alprazolam Tablets USP since market introduction. The majority of these reactions were reported through the medical event voluntary reporting system. Because of the spontaneous nature of the reporting of medical events and the lack of controls, a causal relationship to the use of Alprazolam Tablets USP cannot be readily determined. Reported events include: liver enzyme elevations, hepatitis, hepatic failure, Stevens-Johnson syndrome, hyperprolactinemia, gynecomastia and galactorrhea.
DRUG ABUSE AND DEPENDENCE
Physical and Psychological Dependence
Withdrawal symptoms similar in character to those noted with sedative/hypnotics and alcohol have occurred following discontinuance of benzodiazepines, including Alprazolam Tablets USP. The symptoms can range from mild dysphoria and insomnia to a major syndrome that may include abdominal and muscle cramps, vomiting, sweating, tremors and convulsions. Distinguishing between withdrawal emergent signs and symptoms and the recurrence of illness is often difficult in patients undergoing dose reduction. The long term strategy for treatment of these phenomena will vary with their cause and the therapeutic goal. When necessary, immediate management of withdrawal symptoms requires re-institution of treatment at doses of Alprazolam Tablets USP sufficient to suppress symptoms. There have been reports of failure of other benzodiazepines to fully suppress these withdrawal symptoms. These failures have been attributed to incomplete cross-tolerance but may also reflect the use of inadequate dosing regimen of the substituted benzodiazepine or the effects of concomitant medications.
While it is difficult to distinguish withdrawal and recurrence for certain patients, the time course and the nature of the symptoms may be helpful. A withdrawal syndrome typically includes the occurrence of new symptoms, tends to appear toward the end of taper or shortly after discontinuation, and will decrease with time. In recurring panic disorder, symptoms similar to those observed before treatment may recur either early or late, and they will persist.
While the severity and incidence of withdrawal phenomena appear to be related to dose and duration of treatment, withdrawal symptoms, including seizures, have been reported after only brief therapy with Alprazolam Tablets USP at doses within the recommended range for the treatment of anxiety (eg, 0.75 to 4 mg/day). Signs and symptoms of withdrawal are often more prominent after rapid decrease of dosage or abrupt discontinuance. The risk of withdrawal seizures may be increased at doses above 4 mg/day (see WARNINGS).
Patients, especially individuals with a history of seizures or epilepsy, should not be abruptly discontinued from any CNS depressant agent, including Alprazolam Tablets USP. It is recommended that all patients on Alprazolam Tablets USP who require a dosage reduction be gradually tapered under close supervision (see WARNINGS and DOSAGE AND ADMINISTRATION).
Psychological dependence is a risk with all benzodiazepines, including Alprazolam Tablets USP. The risk of psychological dependence may also be increased at doses greater than 4 mg/day and with longer term use, and this risk is further increased in patients with a history of alcohol or drug abuse. Some patients have experienced considerable difficulty in tapering and discontinuing from Alprazolam Tablets USP, especially those receiving higher doses for extended periods. Addiction-prone individuals should be under careful surveillance when receiving Alprazolam Tablets USP. As with all anxiolytics, repeat prescriptions should be limited to those who are under medical supervision.
Controlled Substance Class
Alprazolam is a controlled substance under the Controlled Substance Act by the Drug Enforcement Administration and Alprazolam Tablets USP have been assigned to Schedule IV.
OVERDOSAGE
Manifestations of alprazolam overdosage include somnolence, confusion, impaired coordination, diminished reflexes and coma. Death has been reported in association with overdoses of alprazolam by itself, as it has with other benzodiazepines. In addition, fatalities have been reported in patients who have overdosed with a combination of a single benzodiazepine, including alprazolam, and alcohol; alcohol levels seen in some of these patients have been lower than those usually associated with alcohol-induced fatality.
The acute oral LD 50 in rats is 331-2171 mg/kg. Other experiments in animals have indicated that cardiopulmonary collapse can occur following massive intravenous doses of alprazolam (over 195 mg/kg; 975 times the maximum recommended daily human dose of 10 mg/day). Animals could be resuscitated with positive mechanical ventilation and the intravenous infusion of norepinephrine bitartrate.
Animal experiments have suggested that forced diuresis or hemodialysis are probably of little value in treating overdosage.
General Treatment of Overdose
Overdosage reports with Alprazolam Tablets USP are limited. As in all cases of drug overdosage, respiration, pulse rate, and blood pressure should be monitored. General supportive measures should be employed, along with immediate gastric lavage. Intravenous fluids should be administered and an adequate airway maintained. If hypotension occurs, it may be combated by the use of vasopressors. Dialysis is of limited value. As with the management of intentional overdosing with any drug, it should be borne in mind that multiple agents may have been ingested. Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdosage. Patients treated with flumazenil should be monitored for re-sedation, respiratory depression, and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert including CONTRAINDICATIONS, WARNINGS and PRECAUTIONS should be consulted prior to use.
ALPRAZOLAM DOSAGE AND ADMINISTRATION
Dosage should be individualized for maximum beneficial effect. While the usual daily dosages given below will meet the needs of most patients, there will be some who require doses greater than 4 mg/day. In such cases, dosage should be increased cautiously to avoid adverse effects.
Anxiety Disorders and Transient Symptoms of Anxiety
Treatment for patients with anxiety should be initiated with a dose of 0.25 to 0.5 mg given three times daily. The dose may be increased to achieve a maximum therapeutic effect, at intervals of 3 to 4 days, to a maximum daily dose of 4 mg, given in divided doses. The lowest possible effective dose should be employed and the need for continued treatment reassessed frequently. The risk of dependence may increase with dose and duration of treatment.
In elderly patients, in patients with advanced liver disease or in patients with debilitating disease, the usual starting dose is 0.25 mg, given two or three times daily. This may be gradually increased if needed and tolerated. The elderly may be especially sensitive to the effects of benzodiazepines.
If side effects occur at the recommended starting dose, the dose may be lowered. In all patients, dosage should be reduced gradually when discontinuing therapy or when decreasing the daily dosage. Although there are no systematically collected data to support a specific discontinuation schedule, it is suggested that the daily dosage be decreased by no more than 0.5 mg every three days. Some patients may require an even slower dosage reduction.
Panic Disorder
The successful treatment of many panic disorder patients has required the use of Alprazolam Tablets USP at doses greater than 4 mg daily. In controlled trials conducted to establish the efficacy of Alprazolam Tablets USP in panic disorder, doses in the range of 1 to 10 mg daily were used. The mean dosage employed was approximately 5 to 6 mg daily. Among the approximately 1700 patients participating in the panic disorder development program, about 300 received Alprazolam Tablets USP in dosages of greater than 7 mg/day, including approximately 100 patients who received maximum dosages of greater than 9 mg/day. Occasional patients required as much as 10 mg a day to achieve a successful response.
Generally, therapy should be initiated at a low dose to minimize the risk of adverse responses in patients especially sensitive to the drug. Thereafter, the dose can be increased at intervals equal to at least 5 times the elimination half-life (about 11 hours in young patients, about 16 hours in elderly patients). Longer titration intervals should probably be used because the maximum therapeutic response may not occur until after the plasma levels achieve steady state. Dose should be advanced until an acceptable therapeutic response (ie, a substantial reduction in or total elimination of panic attacks) is achieved, intolerance occurs, or the maximum recommended dose is attained. For patients receiving doses greater than 4 mg/day, periodic reassessment and consideration of dosage reduction is advised. In a controlled postmarketing dose-response study, patients treated with doses of Alprazolam Tablets USP greater than 4 mg/day for three months were able to taper to 50% of their total maintenance dose without apparent loss of clinical benefit. Because of the danger of withdrawal, abrupt discontinuation of treatment should be avoided. (See WARNINGS, PRECAUTIONS, DRUG ABUSE AND DEPENDENCE).
The following regimen is one that follows the principles outlined above
Treatment may be initiated with a dose of 0.5 mg three times daily. Depending on the response, the dose may be increased at intervals of 3 to 4 days in increments of no more than 1 mg per day. Slower titration to the dose levels greater than 4 mg/day may be advisable to allow full expression of the pharmacodynamic effect of Alprazolam Tablets USP. To lessen the possibility of interdose symptoms, the times of administration should be distributed as evenly as possible throughout the waking hours, that is, on a three or four times per day schedule.
The necessary duration of treatment for panic disorder patients responding to Alprazolam Tablets USP is unknown. After a period of extended freedom from attacks, a carefully supervised tapered discontinuation may be attempted, but there is evidence that this may often be difficult to accomplish without recurrence of symptoms and/or the manifestation of withdrawal phenomena.
In any case, reduction of dose must be undertaken under close supervision and must be gradual. If significant withdrawal symptoms develop, the previous dosing schedule should be reinstituted and, only after stabilization, should a less rapid schedule of discontinuation be attempted. In a controlled postmarketing discontinuation study of panic disorder patients which compared this recommended taper schedule with a slower taper schedule, no difference was observed between the groups in the proportion of patients who tapered to zero dose; however, the slower schedule was associated with a reduction in symptoms associated with a withdrawal syndrome. It is suggested that the dose be reduced by no more than 0.5 mg every three days, with the understanding that some patients may benefit from an even more gradual discontinuation. Some patients may prove resistant to all discontinuation regimens.
HOW SUPPLIED
Alprazolam Tablets USP 0.25 mg are available for oral administration as white, oval shaped, scored tablets, imprinted “ALP” bisect “0.25" on one side and “APO” on the other side. They are supplied as follows:
Bottles of 30 (NDC 60505-1331-3)
Bottles of 60 (NDC 60505-1331-6)
Bottles of 100 (NDC 60505-1331-1)
Bottles of 500 (NDC 60505-1331-5)
Bottles of 1000 (NDC60505-1331-8)
Alprazolam Tablets USP 0.5 mg are available for oral administration as peach, oval shaped, scored tablets, imprinted “ALP” bisect “0.5" on one side and “APO” on the other side. They are supplied as follows:
Bottles of 30 (NDC 60505-1332-3)
Bottles of 60 (NDC 60505-1332-6)
Bottles of 100 (NDC 60505-1332-1)
Bottles of 500 (NDC 60505-1332-5)
Bottles of 1000 (NDC 60505-1332-8)
Alprazolam Tablets USP 1 mg are available for oral administration as light blue, oval shaped, scored tablets, imprinted “ALP” bisect “1" on one side and “APO” on the other side. They are supplied as follows:
Bottles of 30 (NDC 60505-1333-3)
Bottles of 60 (NDC 60505-1333-6)
Bottles of 100 (NDC 60505-1333-1)
Bottles of 500 (NDC 60505-1333-5)
Bottles of 1000 (NDC 60505-1333-8)
Alprazolam Tablets USP 2 mg are available for oral administration as white, rectangle shaped , scored tablets, imprinted “A” bisect “L” partial bisect “P” bisect “R” on one side and “A” bisect “P” partial bisect “O” bisect “2” on the other side. They are supplied as follows:
Bottles of 30 (NDC 60505-1334-3)
Bottles of 60 (NDC 60505-1334-6)
Bottles of 100 (NDC 60505-1334-1)
Bottles of 500 (NDC 60505-1334-5)
Bottles of 1000 (NDC 60505-1334-8)
Store at 20° to 25° C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Dispense in a tight, light-resistant container [see USP].
ANIMAL STUDIES
When rats were treated with alprazolam at 3, 10, and 30 mg/kg/day (15 to 150 times the maximum recommended human dose) orally for 2 years, a tendency for a dose related increase in the number of cataracts was observed in females and a tendency for a dose related increase in corneal vascularization was observed in males. These lesions did not appear until after 11 months of treatment.
CLINICAL STUDIES
Anxiety Disorders
Alprazolam Tablets USP were compared to placebo in double blind clinical studies (doses up to 4 mg/day) in patients with a diagnosis of anxiety or anxiety with associated depressive symptomatology. Alprazolam Tablets USP was significantly better than placebo at each of the evaluation periods of these four week studies as judged by the following psychometric instruments: Physician's Global Impressions, Hamilton Anxiety Rating Scale, Target Symptoms, Patient's Global Impressions and Self-Rating Symptom Scale.
Panic Disorder
Support for the effectiveness of Alprazolam Tablets USP in the treatment of panic disorder came from three short-term, placebo-controlled studies (up to 10 weeks) in patients with diagnoses closely corresponding to DSM-III-R criteria for panic disorder.
The average dose of Alprazolam Tablets USP was 5-6 mg/day in two of the studies, and the doses of Alprazolam Tablets USP were fixed at 2 and 6 mg/day in the third study. In all three studies, Alprazolam Tablets USP was superior to placebo on a variable defined as "the number of patients with zero panic attacks" (range, 37-83% met this criterion), as well as on a global improvement score. In two of the three studies, Alprazolam Tablets USP was superior to placebo on a variable defined as "change from baseline on the number of panic attacks per week" (range, 3.3-5.2), and also on a phobia rating scale. A subgroup of patients who were improved on Alprazolam Tablets USP during short-term treatment in one of these trials was continued on an open basis up to eight months, without apparent loss of benefit.
APOTEX INC.
ALPRAZOLAM TABLETS USP
0.25 mg, 0.50 mg, 1 mg and 2 mg
Manufactured by:
Apotex Inc. Toronto, Ontario, Canada
M9L 1T9
Manufactured for:
Apotex Corp. Weston, Florida,
33326
Revised: March 2006
Rev.0
AlprazolamAlprazolam TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AlprazolamAlprazolam TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AlprazolamAlprazolam TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AlprazolamAlprazolam TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||