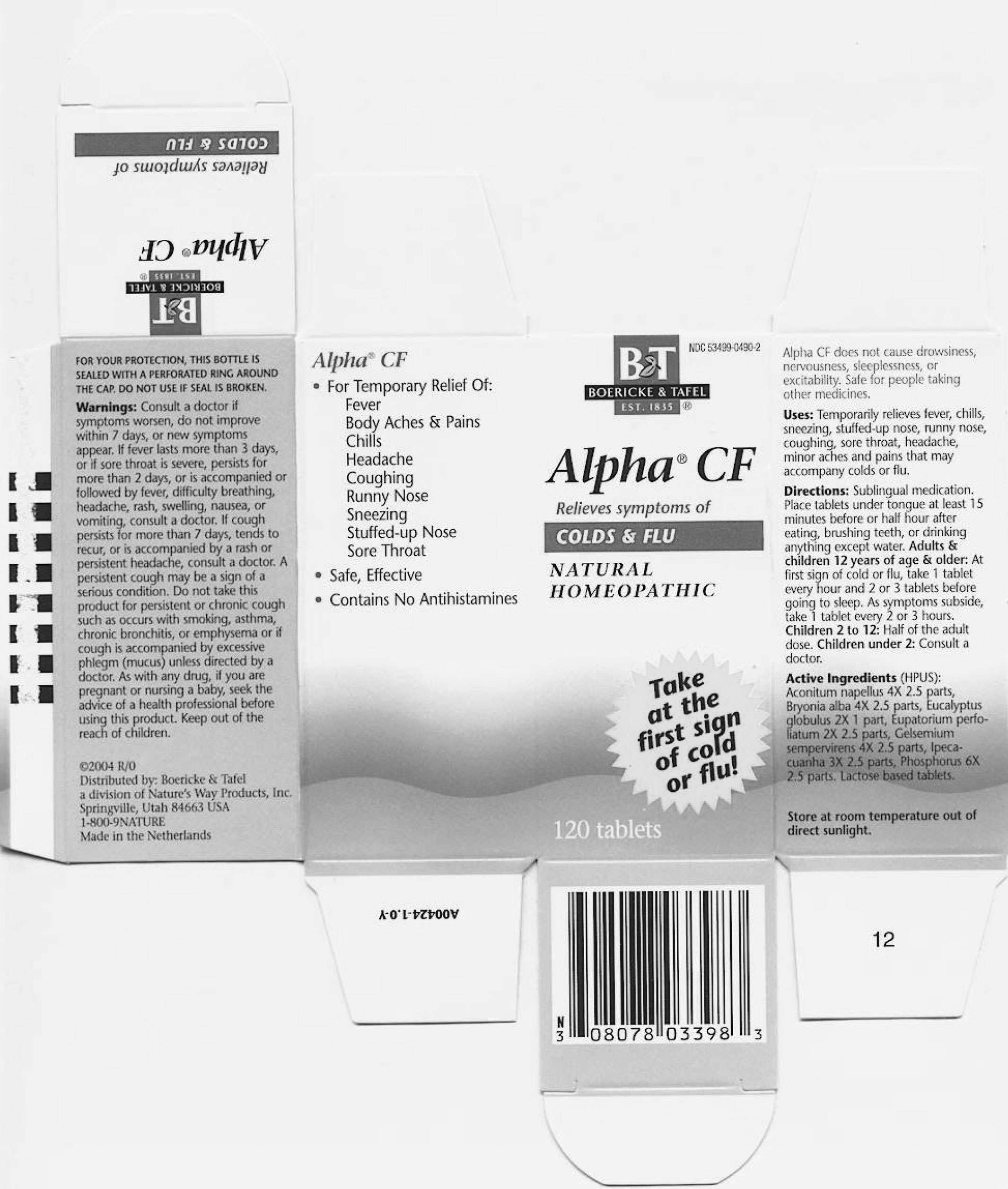
ALPHA CF
Alpha CF
FULL PRESCRIBING INFORMATION
Active ingredient
ACTIVE INGREDIENTS HPUS:
Aconitum napellus 4X
Bryonia alba 4X
Eucalyptus globulus 2X
Eupatorium perfoliatum 2X
Gelsemium sempervirens 4X
Ipecacuanha 3X
Phosphorus 6X
INACTIVE INGREDIENT:
Purpose
PURPOSE:
Keep out of the reach of children
Uses
INDICATIONS AND USAGE:
Temporarily
relieves fever, chills, sneezing, stuffed-up nose, runny nose,
coughing, sore throat, headache, minor aches and pains that may
accompany colds or flu.
Directions:
Sublingual medication. Place
tablets under tongue at least 15 minutes before or half hour after eating, brushing teeth, or drinking anything except water.
WARNINGS:
DOSAGE:
Adults and children 12 years of age and older: At first sign of cold or flu, take 1 tablet every hour and 2 or 3 tablets before gong to sleep. As symptoms subside, take 1 tablet every 2 or 3 hours.
Children 2 to 12: Half of the adult dose.
Children under 2: Consult a doctor
Alpha CF 21803398 Carton
ALPHA CFACONITUM NAPELLUS, BRYONIA ALBA ROOT, EUCALYPTUS GLOBULUS LEAF, EUPATORIUM PERFOLIATUM FLOWERING TOP, GELSEMIUM SEMPERVIRENS ROOT,IPECAC TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||