Alfuzosin Hydrochloride
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use alfuzosin hydrochloride safely and effectively. See full prescribing information for alfuzosin hydrochloride extended-release tablets. Alfuzosin Hydrochloride Extended-Release TabletsInitial U.S. Approval: 2003 RECENT MAJOR CHANGES1.15.45.7INDICATIONS AND USAGE11.11.18.412.3DOSAGE AND ADMINISTRATION2212.3DOSAGE FORMS AND STRENGTHS3CONTRAINDICATIONS Moderate or severe hepatic impairment (4, 8.7, 12.3) Coadministration with potent CYP3A4 inhibitors (e.g. ketoconazole, itraconazole, ritonavir) (4, 5.4, 7.1, 12.3) Known hypersensitivity (e.g., urticaria or angioedema) to alfuzosin or any of the ingredients (4, 6.2) WARNINGS AND PRECAUTIONS Postural hypotension/syncope: Care should be taken in patients with symptomatic hypotension or who have had a hypotensive response to other medications or are concomitantly treated with antihypertensive medication or nitrates (5.1) Use with caution in patients with severe renal impairment (creatinine clearance
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 ALFUZOSIN HYDROCHLORIDE INDICATIONS AND USAGE
- 2 ALFUZOSIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 ALFUZOSIN HYDROCHLORIDE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 ALFUZOSIN HYDROCHLORIDE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 ALFUZOSIN HYDROCHLORIDE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Important Limitations of Use
Alfuzosin
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
Alfuzosin hydrochloride extended-release tablets are contraindicated for use:
- in patients with moderate or severe hepatic impairment (Childs-Pugh categories B and C), since alfuzosin blood levels are increased in these patients. [s ee Use in Specific Populations (8.7) and Clinical Pharmacology ( 12.3 )].
- with potent CYP3A4 inhibitors such as ketoconazole, itraconazole, and ritonavir, since alfuzosin blood levels are increased. [see Drug Interactions (7.1) and Clinical Pharmacology (12.3 )].
- in patients with known hypersensitivity, such as urticaria and angioedema, to alfuzosin hydrochloride or any component of alfuzosin hydrochloride extended-release tablets [see Adverse Reactions (6.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Postural Hypotension
5.2 Patients with Renal Impairment
(creatinine clearance < 30 mL/min) [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]
5.3 Patients with Hepatic Impairment
[see Contraindications (4), Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)]. [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)]
5.4 Drug-Drug Interactions
Potent CYP3A4 Inhibitors[see Contraindications (4), Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
Other alpha adrenergic antagonists: [see Drug Interactions (7.2)].
Phosphodiesterase-5 (PDE5) Inhibitors: [see Drug Interactions (7.4)].
5.5 Prostatic Carcinoma
5.6 Intraoperative Floppy Iris Syndrome (IFIS)
5.7 Priapism
Rarely [see Adverse Reactions (6.2) and Patient Counseling Information [17.3])
5.8 Coronary Insufficiency
5.9 Patients with Congenital or Acquired QT Prolongation
[see Clinical Pharmacology (12.2)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
| Adverse Reaction | Placebo | Alfuzosin Hydrochloride Extended-Release Tablets |
|---|---|---|
| |
(n=678)
|
(n=473)
|
| Dizziness |
19 (2.8%) |
27 (5.7%) |
| Upper respiratory tract infection |
4 (0.6%) |
14 (3%) |
| Headache |
12 (1.8%) |
14 (3%) |
| Fatigue |
12 (1.8%) |
13 (2.7%) |
Body as a whole:
Gastrointestinal system:
Reproductive system:
Respiratory system:
| Symptoms | Placebo | Alfuzosin Hydrochloride Extended-Release Tablets |
|---|---|---|
| |
(n=678)
|
(n=473)
|
| Dizziness |
19 (2.8%) |
27 (5.7%) |
| Hypotension or postural hypotension |
0 |
2 (0.4%) |
| Syncope |
0 |
1 (0.2%) |
6.2 Postmarketing Experience
General disorders:
Cardiac disorders:
Gastrointestinal disorders:
Hepatobiliary disorders:
Respiratory system disorders:
Reproductive system disorders:
Skin and subcutaneous tissue disorders:
Vascular disorders:
[see Warnings and Precautions (5.6)].
7 DRUG INTERACTIONS
7.1 CYP3A4 Inhibitors
[see Contraindications (4), Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)]
7.2 Alpha Adrenergic Antagonists
[see Warnings and Precautions (5.4)].
7.3 Antihypertensive Medication and Nitrates
[see Warnings and Precautions (5.1)]
7.4 PDE5 Inhibitors
[see Warnings and Precautions (5.4)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
.
8.4 Pediatric Use
8.5 Geriatric Use
[see Clinical Pharmacology (12.3)]
8.6 Renal Impairment
[see Clinical Pharmacology (12.3)][see Warnings and Precautions (5.2)]
8.7 Hepatic Impairment
[see Contraindications (4), Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)]
10 OVERDOSAGE
11 DESCRIPTION
192754
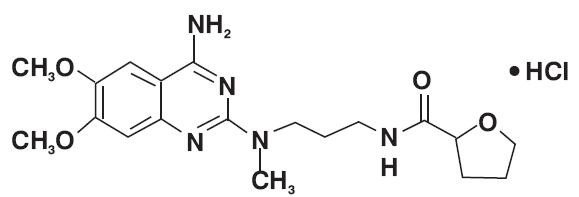
pregelatinized starch
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
1
12.2 Pharmacodynamics
| Drug/Dose | QT | Fridericia method |
Population- specific method |
Subject- specific method |
|---|---|---|---|---|
|
Alfuzosin
10 mg |
-5.8 (-10.2, -1.4) |
4.9 (0.9, 8.8) |
1.8 (-1.4, 5) |
1.8 (-1.3, 5) |
|
Alfuzosin
40 mg |
-4.2 (-8.5, 0.2) |
7.7 (1.9, 13.5) |
4.2 (-0.6, 9) |
4.3 (-0.5, 9.2) |
Moxifloxacin |
6.9 (2.3, 11.5) |
12.7 (8.6, 16.8) |
11 (7, 15) |
11.1 (7.2, 15) |
12.3 Pharmacokinetics
max0-24
Effect of Food
[see Dosage and Administration (2)]
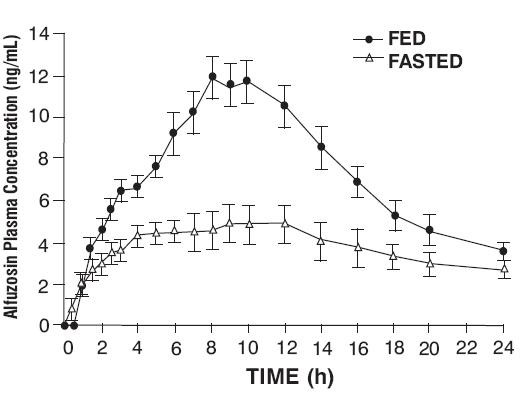
Figure 1 – Mean (SEM) Alfuzosin Plasma Concentration-Time Profiles after a Single Administration of Alfuzosin Hydrochloride Extended-Release 10 mg Tablets to 8 Healthy Middle-Aged Male Volunteers in Fed and Fasted States
in vitro
14
Geriatric Use
Renal ImpairmentCRCRCRCRmax[see Warnings and Precautions (5.2) and Use in Specific Populations (8.6)]
Hepatic Impairment[see Contraindications (4), Warnings and Precautions (5.3) and Use in Specific Populations (8.7)]
Pediatric Use:[see Indications and Usage (1.1) and Use in Specific Populations (8.4)]
maxlast
maxlast
[see Contraindications (4), Warnings and Precautions (5.4) and Drug Interactions (7.1)]
Diltiazem: max0-24max0-12[see Warnings and Precautions (5.1)]
Warfarin:
Digoxin:
Cimetidine: max
Atenolol: maxmax[see Warnings and Precautions (5.1)].
Hydrochlorothiazide:
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no evidence of a drug-related increase in the incidence of tumors in mice following dietary administration of 100 mg/kg/day alfuzosin for 98 weeks (13 and 15 times the maximum recommended human dose [MRHD] of 10 mg based on AUC of unbound drug), in females and males, respectively. The highest dose tested in female mice may not have constituted a maximally tolerated dose. Likewise, there was no evidence of a drug-related increase in the incidence of tumors in rats following dietary administration of 100 mg/kg/day alfuzosin for 104 weeks (53 and 37 times the MRHD in females and males, respectively).
Alfuzosin showed no evidence of mutagenic effect in the Ames and mouse lymphoma assays, and was free of any clastogenic effects in the Chinese hamster ovary cell and in vivo mouse micronucleus assays. Alfuzosin treatment did not induce DNA repair in a human cell line.
There was no evidence of reproductive organ toxicity when male rats were administered oral doses of several hundred times (250 mg/kg/day for 26 weeks) the MRHD of alfuzosin. No impairment of fertility was observed following oral (gavage) administration to male rats at doses of up to 125 mg/kg/day for 70 days. Estrous cycling was inhibited in rats and dogs at approximately 12 and 18 times the MRHD respectively (doses of 25 mg/kg and 20 mg/kg, respectively), but did not result in impaired fertility in female rats.
14 CLINICAL STUDIES
| Trial 1 | Trial 2 | Trial 3 | ||||
|---|---|---|---|---|---|---|
| Symptom Score | Placebo (n = 167) |
Alfuzosin Hydrochloride Extended-Release Tablets 10 mg (n = 170) |
Placebo (n = 152) |
Alfuzosin Hydrochloride Extended-Release Tablets 10 mg (n = 137) | Placebo (n = 150) |
Alfuzosin Hydrochloride Extended-Release Tablets 10 mg (n = 151) |
| Total symptom score
|
||||||
| Baseline |
18.2 (6.4) |
18.2 (6.3) |
17.7 (4.1) |
17.3 (3.5) |
17.7 (5) |
18 (5.4) |
Change |
-1.6 (5.8) |
-3.6 (4.8) |
-4.9 (5.9) |
-6.9 (4.9) |
-4.6 (5.8) |
-6.5 (5.2) |
| p-value |
0.001 |
0.002 |
0.007 |
|||
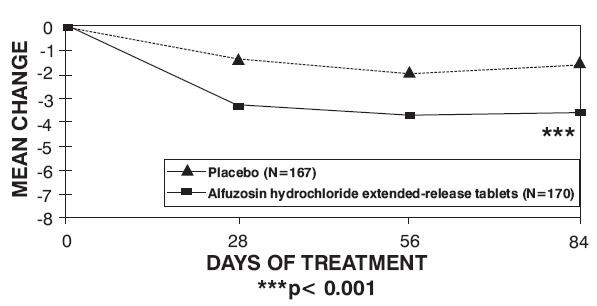
Figure 2 — Mean Change from Baseline in IPSS Total Symptom Score: Trial 1
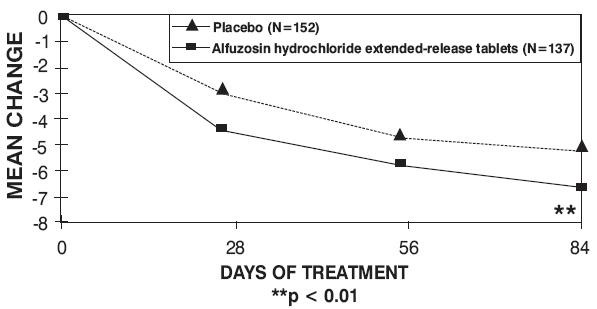
Figure 3 — Mean Change from Baseline in IPSS Total Symptom Score: Trial 2
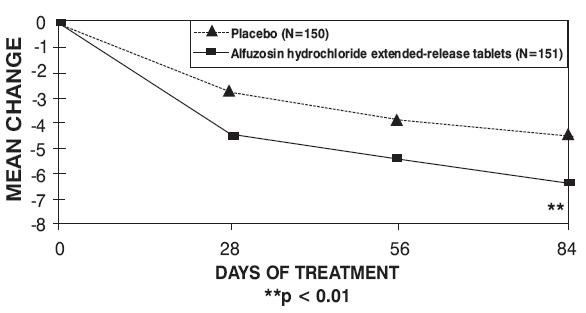
Figure 4 — Mean Change from Baseline in IPSS Total Symptom Score: Trial 3
| Trial 1 | Trial 2 | Trial 3 | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 167) |
Alfuzosin Hydrochloride Extended-Release Tablets 10 mg (n = 170) |
Placebo (n = 147) |
Alfuzosin Hydrochloride Extended-Release Tablets 10 mg (n = 136) |
Placebo (n = 150) |
Alfuzosin Hydrochloride Extended-Release Tablets 10 mg (n = 151) |
|
|
Mean peak flow rate
|
||||||
| Baseline |
10.2 (4) |
9.9 (3.9) |
9.2 (2) |
9.4 (1.9) |
9.3 (2.6) |
9.5 (3) |
Change |
0.2 (3.5) |
1.7 (4.2) |
1.4 (3.2) |
2.3 (3.6) |
0.9 (3) |
1.5 (3.3) |
| p-value |
0.0004 |
0.03 |
0.22 |
|||
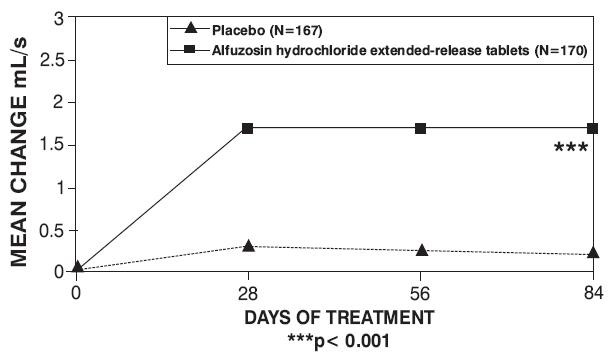
Figure 5 — Mean Change from Baseline in Peak Urine Flow Rate (mL/s): Trial 1

Figure 6 — Mean Change from Baseline in Peak Urine Flow Rate (mL/s): Trial 2
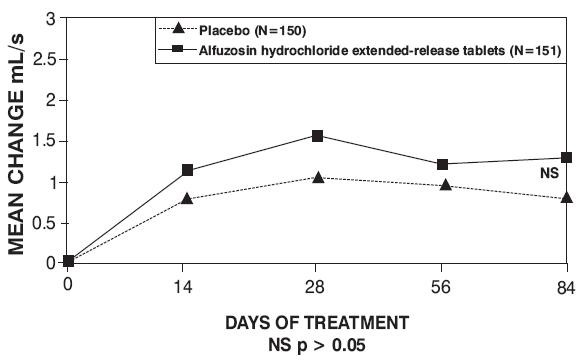
Figure 7 — Mean Change from Baseline in Peak Urine Flow Rate (mL/s): Trial 3
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling.
17.1 Hypotension/Syncope
[see Warnings and Precautions (5.1)]
17.2 Intraoperative Floppy Iris Syndrome
[see Warnings and Precautions (5.6)]
17.3 Priapism
[see Warnings and Precautions (5.7)]
17.4 Instructions of Use
PATIENT INFORMATION
Alfuzosin Hydrochloride Extended-Release Tablets
What is the most important information I should know about alfuzosin hydrochloride extended-release tablets?
Alfuzosin hydrochloride extended-release tablets can cause serious side effects, including a sudden drop in blood pressure, especially when you start treatment.
- Your risk of having this problem may be increased if you take alfuzosin hydrochloride extended-release tablets with certain other medicine that lowers blood pressure:
- medicines for high blood pressure
- a nitrate medicine for angina
Ask your doctor if you are not sure if you are taking one of these medicines.
- Do not drive, operate machinery, or do any dangerous activities until you know how alfuzosin hydrochloride extended-release tablets affect you. This is especially important if you already have a problem with low blood pressure or take medicines to treat high blood pressure.
- If you begin to feel dizzy or lightheaded, lie down with your legs and feet up. If your symptoms do not improve call your doctor.
What is alfuzosin hydrochloride extended-release tablet?
Who should not take alfuzosin hydrochloride extended-release tablets?
- have certain liver problems
- take antifungal medicines like ketoconazole (Nizarol*) or itraconazole (Sporanox*)
- take anti-HIV medicines like ritonavir (Norvir*, Kaletra*)
- are allergic to alfuzosin hydrochloride or any of the ingredients in alfuzosin hydrochloride extended-release tablets. See the end of this leaflet for a complete list of ingredients in alfuzosin hydrochloride extended-release tablets.
Before taking alfuzosin hydrochloride extended-release tablets, tell your doctor if you:
- have liver problems
- have kidney problems
- have had low blood pressure, especially after taking another medicine. Signs of low blood pressure are fainting, dizziness, and lightheadedness.
- have a heart problem called angina
- or any family members have a rare heart condition known as congenital prolongation of the QT interval.
- another alpha blocker medicine
- a medicine to treat high blood pressure
- a medicine to treat angina
- a medicine to treat erectile dysfunction (ED)
- the antifungal medicines like ketoconazole (Nizoral*) or itraconazole (Sporanox*)
- the anti-HIV medicine like , ritonavir (Norvir*, Kaletra*)
What you need to know while taking alfuzosin hydrochloride extended-release tablets?
- If you have an eye surgery for cataract (clouding of the eye) planned, tell your ophthalmologist that you are using alfuzosin hydrochloride extended-release tablets or have previously been treated with an alpha-blocker.
How do I take alfuzosin hydrochloride extended-release tablets?
- Take alfuzosin hydrochloride extended-release tablet exactly as your doctor prescribes it.
- Take alfuzosin hydrochloride extended-release tablet after the same meal each day. Do not take it on an empty stomach.
- Swallow the alfuzosin hydrochloride extended-release tablet whole. Do not crush, split, or chew alfuzosin hydrochloride extended-release tablets.
- If you take too many alfuzosin hydrochloride extended-release tablets call your local poison control center or emergency room right away.
- See “What is the most important information I should know about alfuzosin hydrochloride extended-release tablets?”
- A painful erection that will not go away. Alfuzosin hydrochloride extended-release tablets can cause a painful erection (priapism), which cannot be relieved by having sex. If this happens, get medical help right away. If priapism is not treated, you may not be able to get an erection in the future.
The most common side effects with alfuzosin hydrochloride extended-release tablets are:
- dizziness
- headache
- tiredness
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store alfuzosin hydrochloride extended-release tablets?
- Store alfuzosin hydrochloride extended-release tablets at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F).
- Protect from light and moisture.
General information about alfuzosin hydrochloride extended-release tablets:
What are the ingredients of alfuzosin hydrochloride extended-release tablets?
Active Ingredient:
Inactive Ingredients:
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Ind. Ltd.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 47335-956-83
Alfuzisin Hydrochloride Extended-Release Tablets
10 mg
Rx only
30 TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.
PHARMACIST: PLEASE DISPENSE WITH PATIENT INFORMATION LEAFLET PROVIDED SEPARATELY

Alfuzosin HydrochlorideAlfuzosin Hydrochloride TABLET, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||