Alcaine
Alcon Laboratories, Inc.
Alcon Laboratories, Inc.
FULL PRESCRIBING INFORMATION: CONTENTS*
- ALCAINE DESCRIPTION:
- CLINICAL PHARMACOLOGY:
- ALCAINE INDICATIONS AND USAGE:
- ALCAINE CONTRAINDICATIONS:
- WARNINGS:
- PRECAUTIONS:
- Nursing Mothers:
- Pediatric Use:
- Geriatric Use:
- ALCAINE ADVERSE REACTIONS:
- ALCAINE DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
DESCRIPTION:
ALCAINE® (proparacaine hydrochloride ophthalmic solution, USP) 0.5% is a topical local anesthetic for ophthalmic use. The active ingredient is represented by the structural formula:
Established name:
Proparacaine Hydrochloride
Chemical name:
Benzoic acid, 3-amino-4-propoxy-,2-(diethylamino) ethyl ester, monohydrochloride.
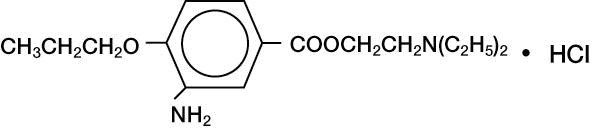
Molecular Weight: 330.85
Each mL contains: Active: proparacaine hydrochloride 5 mg 0.5%. Preservative: benzalkonium chloride (0.01%). Inactives: glycerin and purified water. The pH may be adjusted with hydrochloric acid and/or sodium hydroxide.
CLINICAL PHARMACOLOGY:
ALCAINE® ophthalmic solution is a rapidly-acting topical anesthetic, with induced anesthesia lasting approximately 10-20 minutes.
INDICATIONS AND USAGE:
ALCAINE® ophthalmic solution is indicated for procedures in which a topical ophthalmic anesthetic is indicated: corneal anesthesia of short duration, e.g. tonometry, gonioscopy, removal of corneal foreign bodies, and for short corneal and conjunctival procedures.
CONTRAINDICATIONS:
ALCAINE® ophthalmic solution should be considered contraindicated in patients with known hypersensitivity to any of the ingredients of this preparation.
WARNINGS:
NOT FOR INJECTION - FOR TOPICAL OPHTHALMIC USE ONLY. Prolonged use of a topical ocular anesthetic is not recommended. It may produce permanent corneal opacification with accompanying visual loss.
PRECAUTIONS:
Carcinogenesis, Mutagenesis, Impairment of Fertility.
Long-term studies in animals have not been performed to evaluate carcinogenic potential, mutagenicity, or possible impairment of fertility in males or females.
Pregnancy: Pregnancy Category C:
Animal reproduction studies have not been conducted with ALCAINE® (proparacaine hydrochloride ophthalmic solution, USP) 0.5%. It is also not known whether proparacaine hydrochloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Proparacaine hydrochloride should be administered to a pregnant woman only if clearly needed.
Nursing Mothers:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when proparacaine hydrochloride is administered to a nursing woman.
Pediatric Use:
Safety and effectiveness of proparacaine hydrochloride ophthalmic solution in pediatric patients have been established. Use of proparacaine hydrochloride is supported by evidence from adequate and well-controlled studies in adults and children over the age of twelve, and safety information in neonates and other pediatric patients.
Geriatric Use:
No overall clinical differences in safety or effectiveness have been observed between the elderly and other adult patients.
ADVERSE REACTIONS:
Occasional temporary stinging, burning and conjunctival redness may occur with the use of proparacaine. A rare, severe, immediate-type, apparently hyperallergic corneal reaction characterized by acute, intense and diffuse epithelial keratitis, a gray, ground glass appearance, sloughing of large areas of necrotic epithelium, corneal filaments and, sometimes, iritis with descemetitis has been reported.
Allergic contact dermatitis from proparacaine with drying and fissuring of the fingertips has also been reported.
DOSAGE AND ADMINISTRATION:
Usual Dosage: Removal of foreign bodies and sutures, and for tonometry: 1 to 2 drops (in single instillations) in each eye before operating.
Short corneal and conjunctival procedures: 1 drop in each eye every 5 to 10 minutes for 5 to 7 doses.
NOTE: ALCAINE® (proparacaine hydrochloride ophthalmic solution, USP) 0.5% should be clear to straw-color. If the solution becomes darker, discard the solution.
HOW SUPPLIED:
ALCAINE® (proparacaine hydrochloride ophthalmic solution, USP) 0.5% is supplied in 15 mL DROP-TAINER® dispensers.
NDC 0998-0016-15
Storage:
Bottle must be stored in unit carton to protect contents from light. Store bottles under refrigeration at 2° - 8°C (36° - 46°F).
Rx Only
©2004 Alcon, Inc.
ALCON LABORATORIES, INC.
6201 South Freeway
Fort Worth, Texas 76134 USA
Printed in USA
MedInfo@AlconLabs.com
1-800-757-9195
249039-0609
PRINCIPAL DISPLAY PANEL
NDC 0998-0016-15
Alcon®
Alcaine®
(proparacaine hydrochloride ophthalmic solution USP) 0.5%
15 mL Sterile


Alcaineproparacaine hydrochloride SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||