Albuminuriaforce
Albuminuriaforce
FULL PRESCRIBING INFORMATION
Active ingredient
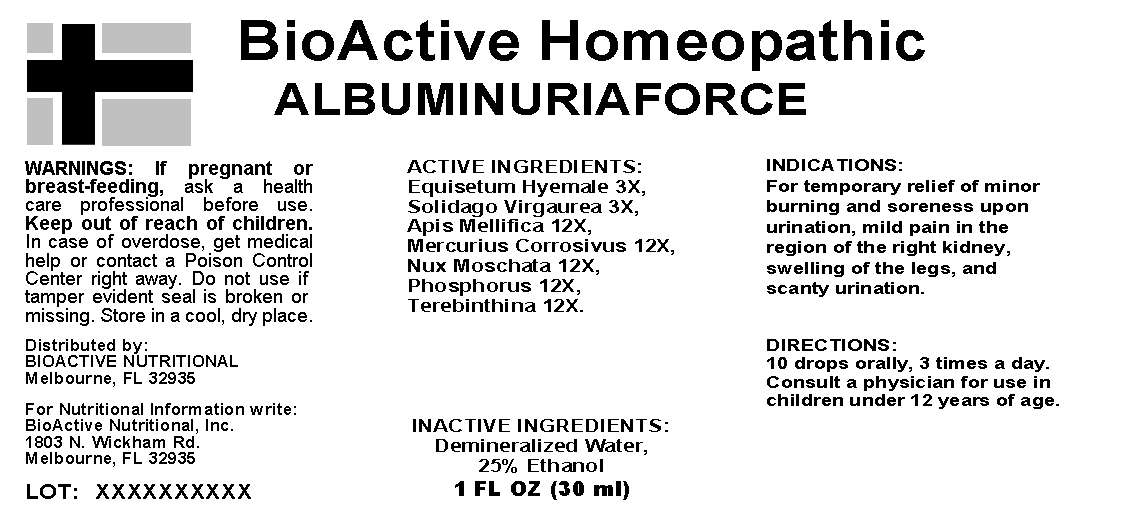
ACTIVE INGREDIENTS: Equisetum hyemale 3X, Solidago virgaurea 3X, Apis Mellifica 12X, Mercurius corrosivus 12X, Nux moschata 12X, Phosphorus 12X, Terebinthina 12X.
Purpose
INDICATIONS: For temporary relief of minor burning and soreness upon urination, mild pain in the region of the right kidney, swelling of the legs, and scanty urination.
WARNINGS: If pregnant or breast-feeding, ask a health care professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away
Do not use if tamper evident seal is broken or missing. Store in a cool, dry place.
DIRECTIONS: 10 drops orally, 3 times a day. Consult a physician for use in children under 12 years of age.
INACTIVE INGREDIENTS: Demineralized water, 25% Ethanol
Distributed By:
BioActive Nutritional
Melbourne, FL 32935
For Nutritional Information Write:
BioActive Nutritional, Inc.
1803 N. Wickham Rd.
Melbourne, FL 32935
BioActive Homeopathic
ALBUMINURIAFORCE
1 FL OZ (30 ML)
AlbuminuriaforceEquisetum hyemale, Solidago virgaurea, Apis mellifica, Mercurius corrosivus, Nux moschata, Phosphorus, Terebinthina, LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||