AHIST
AHIST™
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients (in each immediate-release tablet)
- Purpose
- AHIST Uses
- Warnings
- Directions
- Inactive ingredients
- Questions or Comments?
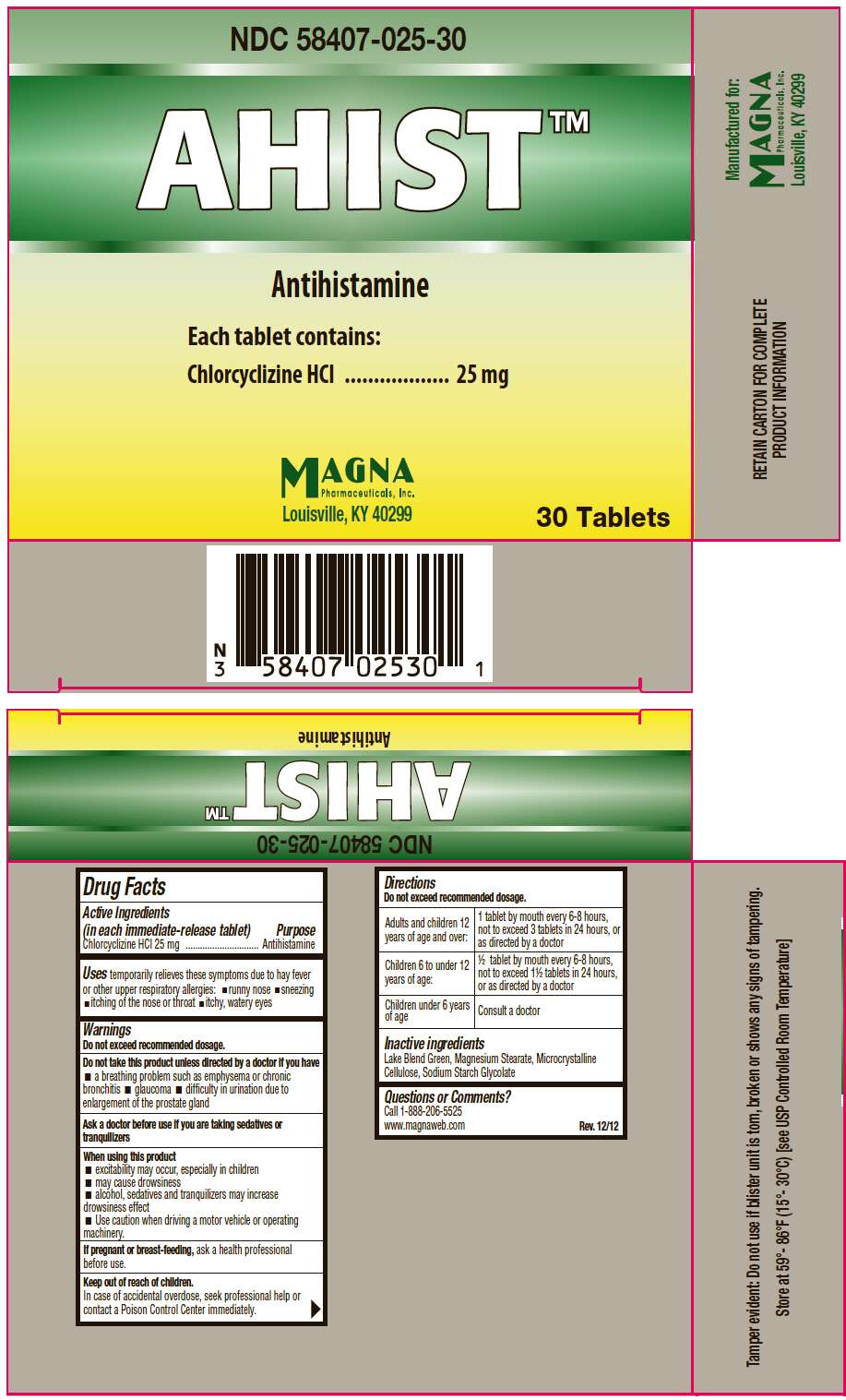
- PRINCIPAL DISPLAY PANEL - 30 Tablet Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredients (in each immediate-release tablet)
Chlorcyclizine HCl 25 mg
Purpose
Antihistamine
AHIST Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
Warnings
Do not exceed recommended dosage.
Do not take this product unless directed by a doctor if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Ask a doctor before use if you are taking sedatives or tranquilizers
When using this product
- excitability may occur, especially in children
- may cause drowsiness
- alcohol, sedatives and tranquilizers may increase drowsiness effect
- Use caution when driving a motor vehicle or operating machinery.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of accidental overdose, seek professional help or contact a Poison Control Center immediately.
Directions
Do not exceed recommended dosage.
| Adults and children 12 years of age and over: | 1 tablet by mouth every 6-8 hours, not to exceed 3 tablets in 24 hours, or as directed by a doctor |
| Children 6 to under 12 years of age: | ½ tablet by mouth every 6-8 hours, not to exceed 1½ tablets in 24 hours, or as directed by a doctor |
| Children under 6 years of age | Consult a doctor |
Inactive ingredients
Lake Blend Green, Magnesium Stearate, Microcrystalline Cellulose, Sodium Starch Glycolate
Questions or Comments?
Call 1-888-206-5525
www.magnaweb.com
Rev. 12/12
Manufactured for:
MAGNA
Pharmaceuticals, Inc.
Louisville, KY 40299
PRINCIPAL DISPLAY PANEL - 30 Tablet Carton
NDC 58407-025-30
AHIST™
Antihistamine
Each tablet contains:
Chlorcyclizine HCl 25 mg
MAGNA
Pharmaceuticals, Inc.
Louisville, KY 40299
30 Tablets
AHISTCHLORCYCLIZINE HYDROCHLORIDE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||