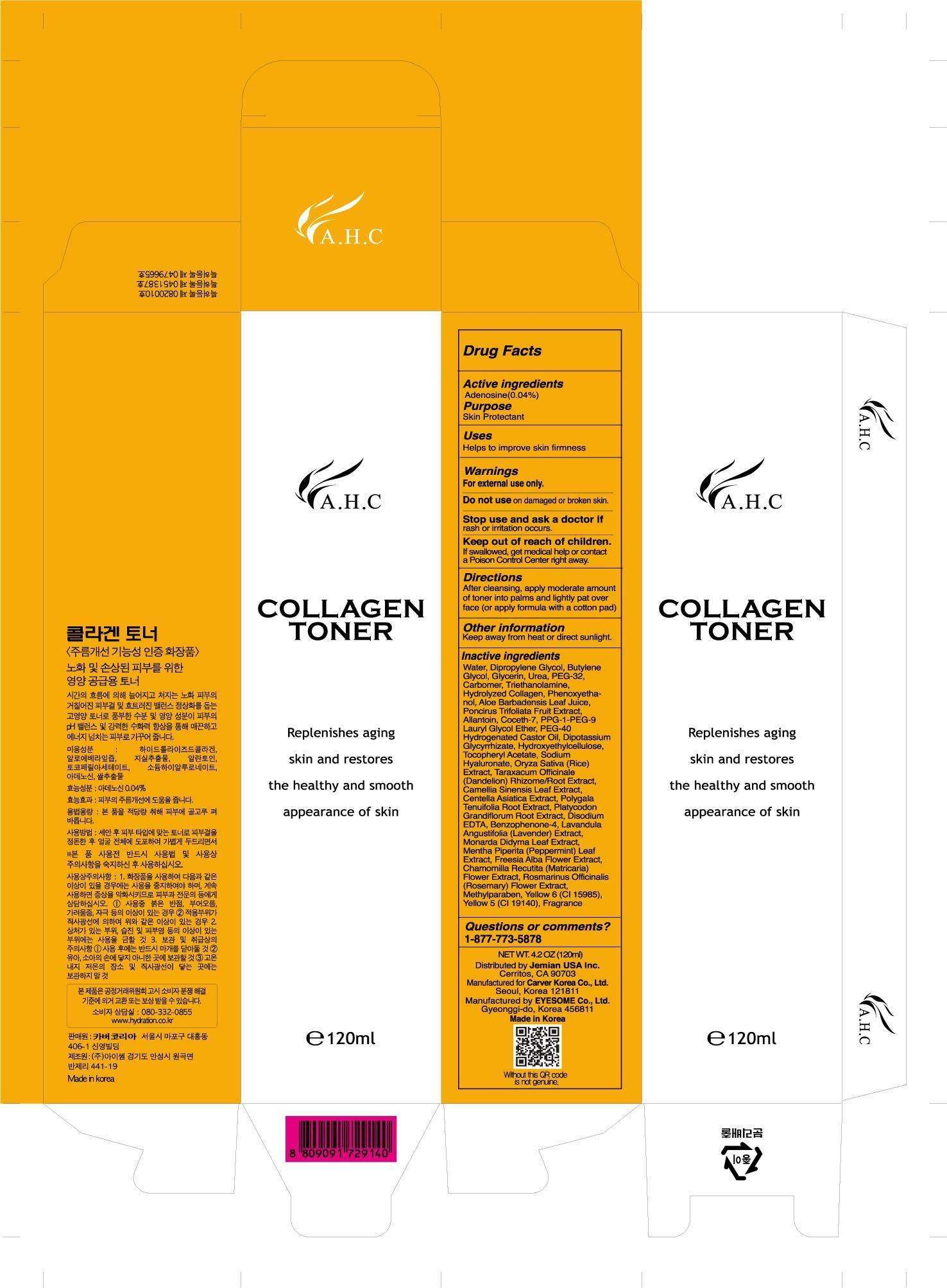
A.H.C. Collagen Toner
Carver Korea Co.,Ltd
Jemian USA Inc.
A.H.C. Collagen Toner
FULL PRESCRIBING INFORMATION: CONTENTS*
- Drug Facts
- A.H.C. Collagen Toner Uses
- Warning
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL - 120mL Bottle Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active Ingredients | Purpose |
| Adenosine 0.04% | Skin Protectant |
A.H.C. Collagen Toner Uses
Help to improve skin natural health
Warning
For external use only
Do not use
on damage or broken skin
Keep out of reach of Children.
Apply small amount to a clean skin and massage gently with fingertips.
Dosage: Small Amount
Administration: apply to a clean skin and massage gently
Inactive Ingredients
WATER
DIPROPYLENE GLYCOL
BUTYLENE GLYCOL
GLYCERIN
UREA
PEG-32 STEARATE
CARBOMER 934
TROLAMINE
HYDROLYSED MARINE COLLAGEN (ENZYMATIC; 2000 MW)
PHENOXYETHANOL
ALOE VERA LEAF
PONCIRUS TRIFOLIATA FRUIT
ALLANTOIN
COCETH-7 CARBOXYLIC ACID
LAURYL GLYCOL HYDROXYPROPYL ETHER
PEG-40 CASTOR OIL
GLYCYRRHIZINATE DIPOTASSIUM
HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%)
.ALPHA.-TOCOPHEROL ACETATE
HYALURONATE SODIUM
ORYZA SATIVA WHOLE
TARAXACUM OFFICINALE ROOT
CAMELLIA SINENSIS WHOLE
CENTELLA ASIATICA
POLYGALA TENUIFOLIA ROOT
PLATYCODON GRANDIFLORUM ROOT
EDETIC ACID
SULISOBENZONE
LAVANDULA ANGUSTIFOLIA WHOLE
MONARDA DIDYMA LEAF
MENTHA PIPERITA LEAF
FREESIA ALBA FLOWER
MATRICARIA CHAMOMILLA WHOLE
ROSMARINUS OFFICINALIS FLOWER
FD&C YELLOW NO. 6
FD&C YELLOW NO. 5
Fragnance
PRINCIPAL DISPLAY PANEL - 120mL Bottle Carton