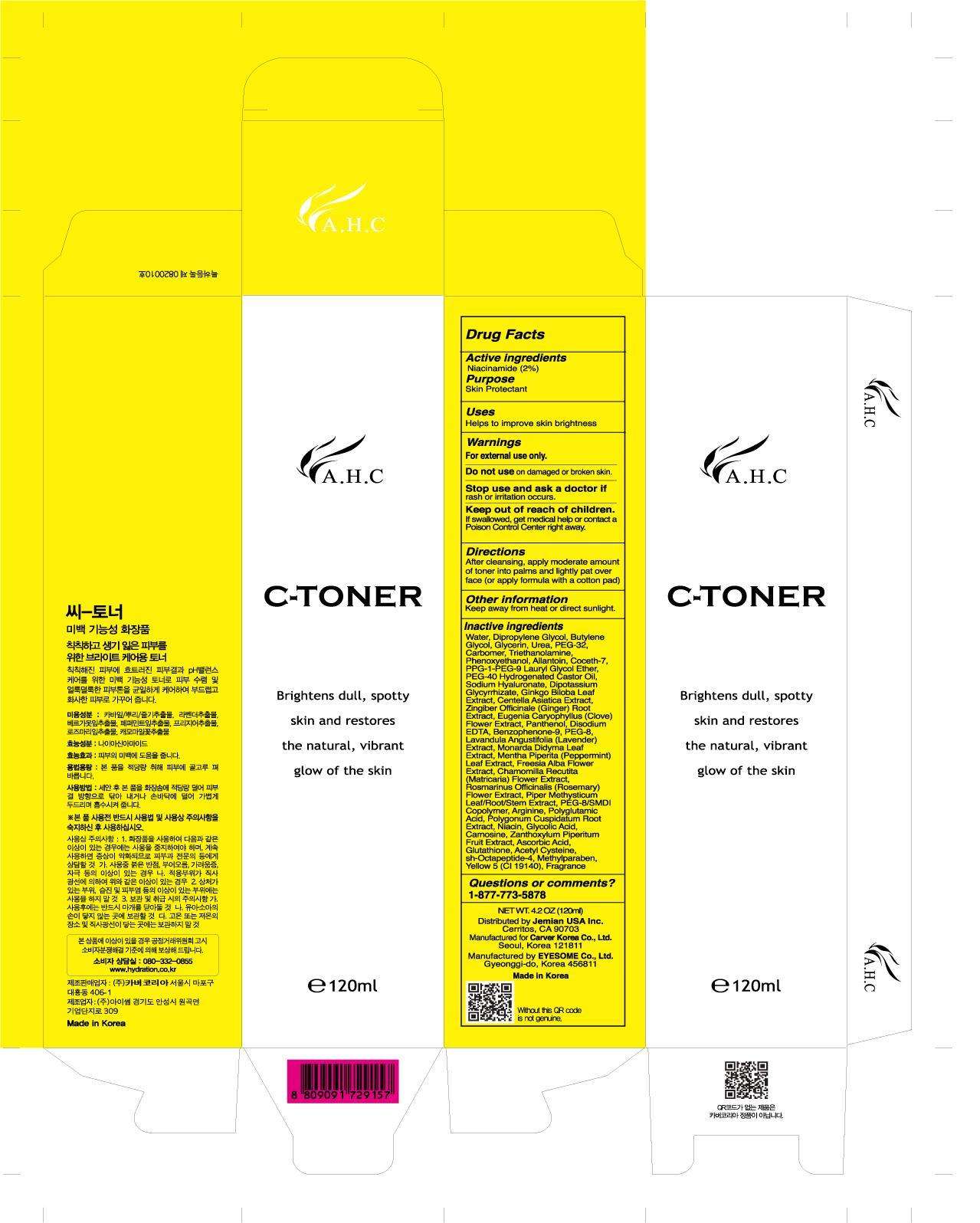
A.H.C. C-TONER
Carver Korea Co.,Ltd
Jemian USA Inc.
A.H.C.C-TONER
FULL PRESCRIBING INFORMATION: CONTENTS*
- Drug Facts
- A.H.C. C-TONER Uses
- Warning
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL - 120mL Bottle Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active Ingredients | Purpose |
| Niacinamide 2% | Skin Protectant |
A.H.C. C-TONER Uses
Help to improve skin brightness
Warning
For external use only
Do not use
on damage or broken skin
Keep out of reach of Children.
Apply small amount to a clean skin and massage gently with fingertips.
Dosage: Small Amount
Administration: apply to a clean skin and massage gently
Inactive Ingredients
WATER
DIPROPYLENE GLYCOL
1,3-BUTYLENE GLYCOL
GLYCERIN
UREA
PEG-32 OLEATE
CARBOMER 934
TRIETHANOLAMINE HYDROCHLORIDE
PHENOXYETHANOL
ALLANTOIN
COCETH-7 CARBOXYLIC ACID
LAURYL GLYCOL HYDROXYPROPYL ETHER
HYDROGENATED CASTOR OIL
HYALURONATE SODIUM
GLYCYRRHIZINATE DIPOTASSIUM
GINKGO BILOBA LEAF OIL
CENTELLA ASIATICA
ZINGIBER OFFICINALE WHOLE
CLOVE
PANTHENOL
ETHYLENEDIAMINETRIACETIC ACID
BENZOPHENONE-9
POLYETHYLENE GLYCOL 300
LAVANDULA ANGUSTIFOLIA WHOLE
MONARDA DIDYMA LEAF
MENTHA PIPERITA LEAF
FREESIA ALBA FLOWER
MATRICARIA CHAMOMILLA FLOWERING TOP OIL
ROSEMARY
PIPER METHYSTICUM WHOLE
PEG-8/SMDI COPOLYMER
ARGININE
GLUTAMIC ACID
POLYGONUM CUSPIDATUM ROOT
NIACIN
GLYCOLIC ACID
CARNOSINE
ZANTHOXYLUM PIPERITUM FRUIT PULP
ASCORBIC ACID
GLUTATHIONE
ACETYLCYSTEINE
ACETYL OCTAPEPTIDE-3
METHYLPARABEN
HC YELLOW NO. 5
PRINCIPAL DISPLAY PANEL - 120mL Bottle Carton