Afeditab
Aphena Pharma Solutions - Tennessee, LLC
Afeditab CR(Nifedipine Extended Release Tablets)
FULL PRESCRIBING INFORMATION: CONTENTS*
- AFEDITAB DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATION AND USAGE
- AFEDITAB CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ADVERSE EXPERIENCES
- OVERDOSAGE
- AFEDITAB DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Repackaging Information
- PRINCIPAL DISPLAY PANEL - 60mg
- PRINCIPAL DISPLAY PANEL - 30mg
FULL PRESCRIBING INFORMATION
Revised: September 2011
Rx only
AFEDITAB DESCRIPTION
Afeditab® CR is an extended release tablet dosage form of the calcium channel blocker nifedipine. Nifedipine is 3,5-pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(2- nitrophenyl)-dimethyl ester, C17H18N2O6, and has the structural formula:
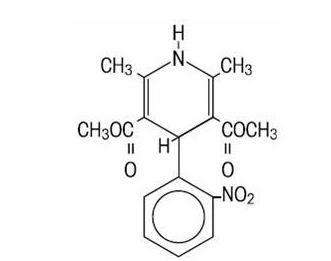
Nifedipine is a yellow crystalline substance, practically insoluble in water but soluble in ethanol. It has a molecular weight of 346.3.
Afeditab® CR tablets contain either 30 mg or 60 mg of nifedipine for once-a-day oral administration.
Each tablet also contains the following inactive ingredients: colloidal silicon dioxide, hypromellose, lactose monohydrate (60 mg), magnesium stearate, and microcrystalline cellulose (30 mg). The inert ingredients in the film coating are: hypromellose, iron oxide, polyethylene glycol, and titanium dioxide. The ingredients of the printing ink are: ammonium hydroxide, iron oxide black, isopropyl alcohol, n-butyl alcohol, propylene glycol and shellac.
Does not meet USP Drug Release Test.
CLINICAL PHARMACOLOGY
Nifedipine is a calcium ion influx inhibitor (slow-channel blocker or calcium ion antagonist) which inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. The contractile processes of vascular smooth muscle and cardiac muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Nifedipine selectively inhibits calcium ion influx across the cell membrane of vascular smooth muscle and cardiac muscle without altering serum calcium concentrations.
Mechanism of Action:
The mechanism by which nifedipine reduces arterial blood pressure involves peripheral arterial vasodilatation and, consequently, a reduction in peripheral vascular resistance. The increased peripheral vascular resistance that is an underlying cause of hypertension results from an increase in active tension in the vascular smooth muscle. Studies have demonstrated that the increase in active tension reflects an increase in cytosolic free calcium.
Nifedipine is a peripheral arterial vasodilator which acts directly on vascular smooth muscle. The binding of nifedipine to voltage-dependent and possibly receptor-operated channels in vascular smooth muscle results in an inhibition of calcium influx through these channels. Stores of intracellular calcium in vascular smooth muscle are limited and thus dependent upon the influx of extracellular calcium for contraction to occur. The reduction in calcium influx by nifedipine causes arterial vasodilation and decreased peripheral vascular resistance which results in reduced arterial blood pressure.
Pharmacokinetics and Metabolism:
Nifedipine is completely absorbed after oral administration. The bioavailability of nifedipine as Afeditab® CR relative to immediate release nifedipine is in the range of 84%-89%. After ingestion of Afeditab® CR tablets under fasting conditions, plasma concentrations peak at about 2.5-5 hours with a second small peak or shoulder evident at approximately 6-12 hours post dose. The elimination half-life of nifedipine administered as Afeditab® CR is approximately 7 hours in contrast to the known 2 hour elimination half-life of nifedipine administered as an immediate release capsule.
When Afeditab® CR is administered as multiples of 30 mg tablets over a dose range of 30 mg to 90 mg, the area under the curve (AUC) is dose proportional: however, the peak plasma concentration for the 90 mg dose given as 3 x 30 mg is 29% greater than predicted from the 30 mg and 60 mg doses.
Two 30 mg Afeditab® CR tablets may be interchanged with a 60 mg Afeditab® CR tablet. Three 30 mg Afeditab CR tablets, however, result in substantially higher Cmax values than those after a single 90 mg Afeditab® CR tablet. Three 30 mg tablets should, therefore, not be considered interchangeable with a 90 mg tablet.
Once daily dosing of nifedipine extended-release tablets under fasting conditions results in decreased fluctuations in the plasma concentration of nifedipine when compared to t.i.d. dosing with immediate-release nifedipine capsules. The mean peak plasma concentration of nifedipine following a 90 mg nifedipine extended-release tablets, administered under fasting conditions, is approximately 115 ng/mL. When nifedipine extended-release tablets is given immediately after a high fat meal in healthy volunteers, there is an average increase of 60% in the peak plasma nifedipine concentration, a prolongation in the time to peak concentration, but no significant change in the AUC. Plasma concentrations of nifedipine when nifedipine extended-release tablets is taken after a fatty meal result in slightly lower peaks compared to the same daily dose of the immediate release formulation administered in three divided doses. This may be, in part, because nifedipine extended-release tablets are less bioavailable than the immediate release formulation.
Nifedipine is extensively metabolized to highly water soluble, inactive metabolites accounting for 60% to 80% of the dose excreted in the urine. Only traces (less than 0.1% of the dose) of the unchanged form can be detected in the urine. The remainder is excreted in the feces in metabolized form, most likely as a result of biliary excretion.
No studies have been performed with nifedipine extended release tablets in patients with renal failure; however, significant alterations in the pharmacokinetics of nifedipine immediate release capsules have not been reported in patients undergoing hemodialysis or chronic ambulatory peritoneal dialysis. Since the absorption of nifedipine from Afeditab® CR could be modified by renal disease, caution should be exercised in treating such patients.
Because hepatic biotransformation is the predominant route for the disposition of nifedipine, its pharmacokinetics may be altered in patients with chronic liver disease. Nifedipine extended-release tablets have not been studied in patients with hepatic disease; however, in patients with hepatic impairment (liver cirrhosis) nifedipine has a longer elimination half-life and higher bioavailability than in healthy volunteers.
The degree of protein binding of nifedipine is high (92%- 98%). Protein binding may be greatly reduced in patients with renal or hepatic impairment.
After administration of nifedipine extended-release tablets to healthy elderly men and women (age > 60 years), the mean Cmax is 36% higher and the average plasma concentration is 70% greater than in younger patients.
In healthy subjects, the elimination half-life of a different sustained release nifedipine formulation was longer in elderly subjects (6.7 h) compared to young subjects (3.8 h) following oral administration. A decreased clearance was also observed in the elderly (348 mL/min) compared to young subjects (519 mL/min) following intravenous administration.
Co-administration of nifedipine with grapefruit juice results in up to a 2-fold increase in AUC and Cmax, due to inhibition of CYP3A4 related first-pass metabolism.
Clinical Studies:
Nifedipine extended-release tablets produced dose-related decreases in systolic and diastolic blood pressure as demonstrated in two double-blind, randomized, placebo-controlled trials in which over 350 patients were treated with nifedipine extended-release tablets 30, 60 or 90 mg once daily for 6 weeks. In the first study, nifedipine extended-release tablets was given as monotherapy and in the second study, nifedipine extended-release tablets was added to a beta-blocker in patients not controlled on a beta-blocker alone. The mean trough (24 hours post-dose) blood pressure results from these studies are shown below:
| *Placebo response subtracted. | ||
| STUDY 1 | ||
| NIFEDIPINE EXTENDED- | ||
| RELEASE TABLETS | MEAN TROUGH | |
| DOSE | N | REDUCTION * |
| 30 MG | 60 | 5.3/2.9 |
| 60 MG | 57 | 8.0/4.1 |
| 90 MG | 55 | 12.5/8.1 |
| STUDY 2 | ||
| NIFEDIPINE EXTENDED- | ||
| RELEASE TABLETS | MEAN TROUGH | |
| DOSE | N | REDUCTION * |
| 30 MG | 58 | 7.6/3.8 |
| 60 MG | 63 | 10.1/5.3 |
| 90 MG | 62 | 10.2/5.8 |
The trough/peak ratios estimated from 24 hour blood pressure monitoring ranged from 41%-78% for diastolic and 46%-91% for systolic blood pressure.
Hemodynamics:
Like other slow-channel blockers, nifedipine exerts a negative inotropic effect on isolated myocardial tissue. This is rarely, if ever, seen in intact animals or man, probably because of reflex responses to its vasodilating effects. In man, nifedipine decreases peripheral vascular resistance which leads to a fall in systolic and diastolic pressures, usually minimal in normotensive volunteers (less than 5 to 10 mm Hg systolic), but sometimes larger. With nifedipine extended-release tablets, these decreases in blood pressure are not accompanied by any significant change in heart rate. Hemodynamic studies of the immediate release nifedipine formulation in patients with normal ventricular function have generally found a small increase in cardiac index without major effects on ejection fraction, left ventricular end-diastolic pressure (LVEDP) or volume (LVEDV). In patients with impaired ventricular function, most acute studies have shown some increase in ejection fraction and reduction in left ventricular filling pressure.
Electrophysiologic Effects:
Although, like other members of its class, nifedipine causes a slight depression of sinoatrial node function and atrioventricular conduction in isolated myocardial preparations, such effects have not been seen in studies in intact animals or in man. In formal electro-physiologic studies, predominantly in patients with normal conduction systems, nifedipine administered as the immediate release capsule has had no tendency to prolong atrioventricular conduction or sinus node recovery time, or to slow sinus rate.
INDICATION AND USAGE
Afeditab® CR is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents.
AFEDITAB CONTRAINDICATIONS
Known hypersensitivity to nifedipine.
WARNINGS
Excessive Hypotension: Although in most patients the hypotensive effect of nifedipine is modest and well tolerated, occasional patients have had excessive and poorly tolerated hypotension. These responses have usually occurred during initial titration or at the time of subsequent upward dosage adjustment, and may be more likely in patients using concomitant beta-blockers.
Severe hypotension and/or increased fluid volume requirements have been reported in patients who received immediate-release capsules together with a beta-blocking agent and who underwent coronary artery bypass surgery using high dose fentanyl anesthesia. The interaction with high dose fentanyl appears to be due to the combination of nifedipine and a beta-blocker, but the possibility that it may occur with nifedipine alone, with low doses of fentanyl, in other surgical procedures, or with other narcotic analgesics cannot be ruled out. In nifedipine-treated patients where surgery using high dose fentanyl anesthesia is contemplated, the physician should be aware of these potential problems and, if the patient’s condition permits, sufficient time (at least 36 hours) should be allowed for nifedipine to be washed out of the body prior to surgery.
Increased Angina and/or Myocardial Infarction: Rarely, patients, particularly those who have severe obstructive coronary artery disease, have developed well-documented increased frequency, duration and/or severity of angina or acute myocardial infarction upon starting nifedipine or at the time of dosage increase. The mechanism of this effect is not established.
Beta-Blocker Withdrawal: When discontinuing a beta-blocker it is important to taper its dose, if possible, rather than stopping abruptly before beginning nifedipine. Patients recently withdrawn from beta-blockers may develop a withdrawal syndrome with increased angina, probably related to increased sensitivity to catecholamines. Initiation of nifedipine treatment will not prevent this occurrence and on occasion has been reported to increase it.
Congestive Heart Failure: Rarely, patients (usually while receiving a beta-blocker) have developed heart failure after beginning nifedipine. Patients with tight aortic stenosis may be at greater risk for such an event, as the unloading effect of nifedipine would be expected to be of less benefit to these patients, owing to their fixed impedance to flow across the aortic valve.
PRECAUTIONS
Because nifedipine decreases peripheral vascular resistance, careful monitoring of blood pressure during the initial administration and titration of Afeditab® CR is suggested. Close observation is especially recommended for patients already taking medications that are known to lower blood pressure (See WARNINGS ).
Mild to moderate peripheral edema occurs in a dose-dependent manner with Afeditab® CR. The placebo subtracted rate is approximately 8% at 30 mg, 12% at 60 mg and 19% at 90 mg daily. This edema is a localized phenomenon, thought to be associated with vasodilation of dependent arterioles and small blood vessels and not due to left ventricular dysfunction or generalized fluid retention. With patients whose hypertension is complicated by congestive heart failure, care should be taken to differentiate this peripheral edema from the effects of increasing left ventricular dysfunction.
Afeditab® CR is an extended-release tablet and should be swallowed whole and taken on an empty stomach. It should not be administered with food. Do not chew, divide or crush tablets.
Patients should be advised that empty matrix “ghosts” (tablets) may pass via colostomy or in the stool, and that this is of no concern since the active medication has already been absorbed.
Rare, usually transient, but occasionally significant elevations of enzymes such as alkaline phosphatase, CPK, LDH, SGOT, and SGPT have been noted. The relationship to nifedipine therapy is uncertain in most cases, but probable in some. These laboratory abnormalities have rarely been associated with clinical symptoms; however, cholestasis with or without jaundice has been reported. A small increase (<5%) in mean alkaline phosphatase was noted in patients treated with nifedipine extended-release tablets. This was an isolated finding and it rarely resulted in values which fell outside the normal range. Rare instances of allergic hepatitis have been reported with nifedipine treatment. In controlled studies, nifedipine extended-release tablets did not adversely affect serum uric acid, glucose, cholesterol or potassium.
Nifedipine, like other calcium channel blockers, decreases platelet aggregation in vitro. Limited clinical studies have demonstrated a moderate but statistically significant decrease in platelet aggregation and increase in bleeding time in some nifedipine patients. This is thought to be a function of inhibition of calcium transport across the platelet membrane. No clinical significance for these findings has been demonstrated.
Positive direct Coombs’ test with or without hemolytic anemia has been reported but a causal relationship between nifedipine administration and positivity of this laboratory test, including hemolysis, could not be determined.
Although nifedipine has been used safely in patients with renal dysfunction and has been reported to exert a beneficial effect in certain cases, rare reversible elevations in BUN and serum creatinine have been reported in patients with pre-existing chronic renal insufficiency. The relationship to nifedipine therapy is uncertain in most cases but probable in some.
Beta-adrenergic blocking agents
(See
WARNINGS
.)
Nifedipine is mainly eliminated by metabolism and is a substrate of CYP3A. Inhibitors and inducers of CYP3A4 can impact the exposure to nifedipine and consequently its desirable and undesirable effects. In vitro and in vivo data indicate that nifedipine can inhibit the metabolism of drugs that are substrates of CYP3A, thereby increasing the exposure to other drugs. Nifedipine is a vasodilator, and co-administration of other drugs affecting blood pressure may result in pharmacodynamic interactions.
Cardiovascular Drugs
Antiarrhythmics
Quinidine: Quinidine is a substrate of CYP3A and has been shown to inhibit CYP3A in vitro. Co-administration of multiple doses of quinidine sulfate, 200 mg t.i.d., and nifedipine, 20 mg t.i.d., increased Cmax and AUC of nifedipine in healthy volunteers by factors of 2.30 and 1.37, respectively. The heart rate in the initial interval after drug administration was increased by up to 17.9 beats/minute. The exposure to quinidine was not importantly changed in the presence of nifedipine. Monitoring of heart rate and adjustment of the nifedipine dose, if necessary, are recommended when quinidine is added to a treatment with nifedipine.
Flecainide: There has been too little experience with the co-administration of TAMBOCOR with nifedipine to recommend concomitant use.
Calcium Channel Blockers
Diltiazem: Pre-treatment of healthy volunteers with 30 mg or 90 mg t.i.d. diltiazem p.o. increased the AUC of nifedipine after a single dose of 20 mg nifedipine by factors of 2.2 and 3.1, respectively. The corresponding Cmax values of nifedipine increased by factors of 2.0 and 1.7, respectively. Caution should be exercised when co-administering diltiazem and nifedipine and a reduction of the dose of nifedipine should be considered.
Verapamil: Verapamil, a CYP3A inhibitor, can inhibit the metabolism of nifedipine and increase the exposure to nifedipine during concomitant therapy. Blood pressure should be monitored and reduction of the dose of nifedipine considered.
ACE Inhibitors
Benazepril: In healthy volunteers receiving single dose of 20 mg nifedipine ER and benazepril 20 mg, the plasma concentrations of benazeprilat and nifedipine in the presence and absence of each other were not statistically significantly different. A hypotensive effect was only seen after co-administration of the two drugs. The tachycardic effect of nifedipine was attenuated in the presence of benazepril.
Angiotensin-II Blockers
Irbesartan:
In vitro studies show significant inhibition of the formation of oxidized irbesartan metabolites by nifedipine. However, in clinical studies, concomitant nifedipine had no effect on irbesartan pharmacokinetics.
Candesartan: No significant drug interaction has been reported in studies with candesartan cilexitil given together with nifedipine. Because candesartan is not significantly metabolized by the cytochrome P450 system and at therapeutic concentrations has no effect on cytochrome P450 enzymes, interactions with drugs that inhibit or are metabolized by those enzymes would not be expected.
Beta-blockers
Nifedipine extended-release tablets was well tolerated when administered in combination with beta-blockers in 187 hypertensive patients in a placebo-controlled clinical trial. However, there have been occasional literature reports suggesting that the combination of nifedipine and beta-adrenergic blocking drugs may increase the likelihood of congestive heart failure, severe hypotension or exacerbation of angina in patients with cardiovascular disease. Clinical monitoring is recommended and a dose adjustment of nifedipine should be considered.
Timolol: Hypotension is more likely to occur if dihydropryridine calcium antagonists such as nifedipine are co-administered with timolol.
Central Alpha1-Blockers
Doxazosin: Healthy volunteers participating in a multiple dose doxazosin-nifedipine interaction study received 2 mg doxazosin q.d. alone or combined with 20 mg nifedipine ER b.i.d. Co-administration of nifedipine resulted in a decrease in AUC and Cmax of doxazosin to 83% and 86% of the values in the absence of nifedipine, respectively. In the presence of doxazosin, AUC and Cmax of nifedipine were increased by factors of 1.13 and 1.23, respectively. Compared to nifedipine monotherapy, blood pressure was lower in the presence of doxazosin. Blood pressure should be monitored when doxazosin is co-administered with nifedipine, and dose reduction of nifedipine considered.
Digitalis
Digoxin: Since there have been isolated reports of patients with elevated digoxin levels, and there is a possible interaction between digoxin and nifedipine, it is recommended that digoxin levels be monitored when initiating, adjusting and discontinuing nifedipine extended-release tablets to avoid possible over- or under-digitalization.
Antithrombotics
Coumarins: There have been rare reports of increased prothrombin time in patients taking coumarin anticoagulants to whom nifedipine was administered. However, the relationship to nifedipine therapy is uncertain.
Platelet Aggregation Inhibitors
Clopidogrel: No clinically significant pharmacodynamic interactions were observed when clopidrogrel was co-administered with nifedipine.
Tirofiban: Co-administration of nifedipine did not alter the exposure to tirofiban importantly.
Non-Cardiovascular Drugs
Antifungal Drugs
Ketoconazole, itraconazole and fluconazole are CYP3A inhibitors and can inhibit the metabolism of nifedipine and increase the exposure to nifedipine during concomitant therapy. Blood pressure should be monitored and a dose reduction of nifedipine considered.
Antisecretory Drugs
Omeprazole: In healthy volunteers receiving a single dose of 10 mg nifedipine, AUC and Cmax of nifedipine after pretreatment with omeprazole 20 mg q.d. for 8 days were 1.26 and 0.87 times those after pre-treatment with placebo. Pretreatment with or co-administration of omeprazole did not impact the effect of nifedipine on blood pressure or heart rate. The impact of omeprazole on nifedipine is not likely to be of clinical relevance.
Pantoprazole: In healthy volunteers the exposure to neither drug was changed significantly in the presence of the other drug.
Ranitidine: Five studies in healthy volunteers investigated the impact of multiple ranitidine doses on the single or multiple dose pharmacokinetics of nifedipine. Two studies investigated the impact of coadministered ranitidine on blood pressure in hypertensive subjects on nifedipine. Co-administration of ranitidine did not have relevant effects on the exposure to nifedipine that affected the blood pressure or heart rate in normotensive or hypertensive subjects.
Cimetidine: Five studies in healthy volunteers investigated the impact of multiple cimetidine doses on the single or multiple dose pharmacokinetics of nifedipine. Two studies investigated the impact of coadministered cimetidine on blood pressure in hypertensive subjects on nifedipine.
In normotensive subjects receiving single doses of 10 mg or multiple doses of up to 20 mg nifedipine t.i.d. alone or together with cimetidine up to 1000 mg/day, the AUC values of nifedipine in the presence of cimetidine were between 1.52 and 2.01 times those in the absence of cimetidine. The Cmax values of nifedipine in the presence of cimetidine were increased by factors ranging between 1.60 and 2.02. The increase in exposure to nifedipine by cimetidine was accompanied by relevant changes in blood pressure or heart rate in normotensive subjects. Hypertensive subjects receiving 10 mg q.d. nifedipine alone or in combination with cimetidine 1000 mg q.d. also experienced relevant changes in blood pressure when cimetidine was added to nifedipine. The interaction between cimetidine and nifedipine is of clinical relevance and blood pressure should be monitored and a reduction of the dose of nifedipine considered.
Antibacterial Drugs
Quinupristin/Dalfopristin:
In vitro drug interaction studies have demonstrated that quinupristin/dalfopristin significantly inhibits the CYP3A metabolism of nifedipine. Concomitant administration of quinupristin/dalfopristin and nifedipine (repeated oral dose) in healthy volunteers increased AUC and Cmax for nifedipine by factors of 1.44 and 1.18, respectively, compared to nifedipine monotherapy. Upon co-administration of quinupristin/dalfopristin with nifedipine, blood pressure should be monitored and a reduction of the dose of nifedipine considered.
Erythromycin: Erythromycin, a CYP3A inhibitor, can inhibit the metabolism of nifedipine and increase the exposure to nifedipine during concomitant therapy. Blood pressure should be monitored and reduction of the dose of nifedipine considered.
Antitubercular Drugs
Rifampin: Pretreatment of healthy volunteers with 600 mg/day rifampin p.o. decreased the exposure to oral nifedipine (20 μg/kg) to 13%. The exposure to intravenous nifedipine by the same rifampin treatment was decreased to 70%. Dose adjustment of nifedipine may be necessary if nifedipine is co-administered with rifampin.
Rifapentine: Rifapentine, as an inducer of CYP3A4, can decrease the exposure to nifedipine. A dose adjustment of nifedipine when co-administered with rifapentine should be considered.
Antiviral Drugs
Amprenavir, atanazavir, delavirine, fosamprinavir, indinavir, nelfinavir
and
ritonavir, as CYP3A inhibitors, can inhibit the metabolism of nifedipine and increase the exposure to nifedipine. Caution is warranted and clinical monitoring of patients recommended.
CNS Drugs
Nefazodone, a CYP3A inhibitor, can inhibit the metabolism of nifedipine and increase the exposure to nifedipine during concomitant therapy. Blood pressure should be monitored and a reduction of the dose of nifedipine considered.
Valproic acid may increase the exposure to nifedipine during concomitant therapy. Blood pressure should be monitored and a dose reduction of nifedipine considered.
Phenytoin: Nifedipine is metabolized by CYP3A4. Co-administration of nifedipine 10 mg capsule and 60 mg nifedipine coat-core tablet with phenytoin, an inducer of CYP3A4, lowered the AUC and Cmax of nifedipine by approximately 70%. When using nifedipine with phenytoin, the clinical response to nifedipine should be monitored and its dose adjusted if necessary.
Phenobarbitone and carbamazepine as inducers of CYP3A can decrease the exposure to nifedipine. Dose adjustment of nifedipine may be necessary if phenobarbitone, carbamazepine or phenytoin is coadministered.
Antiemetic Drugs
Dolasetron: In patients taking dolasetron by the oral or intravenous route and nifedipine, no effect was shown on the clearance of hydrodolasetron.
Immunosuppressive Drugs
Tacrolimus: Nifedipine has been shown to inhibit the metabolism of tacrolimus in vitro. Transplant patients on tacrolimus and nifedipine required from 26% to 38% smaller doses than patients not receiving nifedipine. Nifedipine can increase the exposure to tacrolimus. When nifedipine is co-administered with tacrolimus the blood concentrations of tacrolimus should be monitored and a reduction of the dose of tacrolimus considered.
Sirolimus: A single 60 mg dose of nifedipine and a single 10 mg dose of sirolimus oral solution were administered to 24 healthy volunteers. Clinically significant pharmacokinetic drug interactions were not observed.
Glucose Lowering Drugs
Pioglitazone: Co-administration of pioglitazone for 7 days with 30 mg nifedipine ER administered orally q.d. for 4 days to male and female volunteers resulted in least square mean (90% CI) values for unchanged nifedipine of 0.83 (0.73-0.95) for Cmax and 0.88 (0.80-0.96) for AUC relative to nifedipine monotherapy. In view of the high variability of nifedipine pharmacokinetics, the clinical significance of this finding is unknown.
Rosiglitazone: Co-administration of rosiglitazone (4 mg b.i.d.) was shown to have no clinically relevant effect on the pharmacokinetics of nifedipine.
Metformin: A single dose metformin-nifedipine interaction study in normal healthy volunteers demonstrated that co-administration of nifedipine increased plasma metformin Cmax and AUC by 20% and 9%, respectively, and increased the amount of metformin excreted in urine. Tmax and half-life were unaffected. Nifedipine appears to enhance the absorption of metformin.
Miglitol: No effect of miglitol was observed on the pharmacokinetics and pharmacodynamics of nifedipine.
Repaglinide: Co-administration of 10 mg nifedipine with a single dose of 2 mg repaglinide (after 4 days nifedipine 10 mg t.i.d. and repaglinide 2 mg t.i.d.) resulted in unchanged AUC and Cmax values for both drugs.
Acarbose: Nifedipine tends to produce hyperglycemia and may lead to loss of glucose control. If nifedipine is co-administered with acarbose, blood glucose levels should be monitored carefully and a dose adjustment of nifedipine considered.
Drugs Interfering with Food Absorption
Orlistat: In 17 normal-weight subjects receiving orlistat 120 mg t.i.d. for 6 days, orlistat did not alter the bioavailability of 60 mg nifedipine (extended-release tablets).
Dietary Supplements
Grapefruit Juice: In healthy volunteers, a single dose co-administration of 250 mL double strength grapefruit juice with 10 mg nifedipine increased AUC and Cmax by factors of 1.35 and 1.13, respectively. Ingestion of repeated doses of grapefruit juice (5 x 200 mL in 12 hours) after administration of 20 mg nifedipine ER increased AUC and Cmax of nifedipine by a factor of 2.0. Grapefruit juice should be avoided by patients on nifedipine. The intake of grapefruit juice should be stopped at least 3 days prior to initiating patients on nifedipine.
Herbals
St. John’s Wort: Is an inducer of CYP3A4 and may decrease the exposure to nifedipine. Dose adjustment of nifedipine may be necessary if St. John’s Wort is co-administered.
CYP2D6 Probe Drug
Debrisoquine: In healthy volunteers, pretreatment with nifedipine 20 mg t.i.d. for 5 days did not change the metabolic ratio of hydroxydebrisoquine to debrisoquine measured in urine after a single dose of 10 mg debrisoquine. Thus, it is improbable that nifedipine inhibits in vivo the metabolism of other drugs that are substrates of CYP2D6.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Nifedipine was administered orally to rats for two years and was not shown to be carcinogenic. When given to rats prior to mating, nifedipine caused reduced fertility at a dose approximately 30 times the maximum recommended human dose. There is a literature report of reversible reduction in the ability of human sperm obtained from a limited number of infertile men taking recommended doses of nifedipine to bind to and fertilize an ovum in vitro. In vivo mutagenicity studies were negative.
Pregnancy Category C. In rodents, rabbits and monkeys, nifedipine has been shown to have a variety of embryotoxic, placentotoxic and fetotoxic effects, including stunted fetuses (rats, mice and rabbits), digital anomalies (rats and rabbits), rib deformities (mice), cleft palate (mice), small placentas and underdeveloped chorionic villi (monkeys), embryonic and fetal deaths (rats, mice and rabbits), prolonged pregnancy (rats; not evaluated in other species), and decreased neonatal survival (rats; not evaluated in other species). On a mg/kg or mg/m2 basis, some of the doses associated with these various effects are higher than the maximum recommended human dose and some are lower, but all are within an order of magnitude of it.
The digital anomalies seen in nifedipine-exposed rabbit pups are strikingly similar to those seen in pups exposed to phenytoin, and these are in turn similar to the phalangeal deformities that are the most common malformation seen in human children with in utero exposure to phenytoin.
There are no adequate and well-controlled studies in pregnant women. Nifedipine should generally be avoided during pregnancy and used only if the potential benefit justifies the potential risk to the fetus.
Nifedipine is excreted in human milk. Therefore, a decision should be made to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Although small pharmacokinetic studies have identified an increased half-life and increased Cmax and AUC (See CLINICAL PHARMACOLOGY: Pharmacokinetics and Metabolism ), clinical studies of nifedipine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE EXPERIENCES
The incidence of adverse events during treatment with nifedipine extended-release tablets in doses up to 90 mg daily were derived from multi-center placebo-controlled clinical trials in 370 hypertensive patients. Atenolol 50 mg once daily was used concomitantly in 187 of the 370 patients on nifedipine extended-release tablets and in 64 of the 126 patients on placebo. All adverse events reported during nifedipine extended-release tablets therapy were tabulated independently of their causal relationship to medication.
The most common adverse event reported with nifedipine extended-release tablets was peripheral edema. This was dose related and the frequency was 18% on nifedipine extended-release tablets 30 mg daily, 22% on nifedipine extended-release tablets 60 mg daily and 29% on nifedipine extended-release tablets 90 mg daily versus 10% on placebo.
Other common adverse events reported in the above placebo-controlled trials include:
| NIFEDIPINE EXTENDED- | ||
| RELEASE TABLETS (%) | PLACEBO (%) | |
| (n=370) | (n=126) | |
| Adverse Event | ||
| Headache | 19 | 13 |
| Flushing/heat | 4 | 0 |
| Dizziness | 4 | 2 |
| Fatigue/asthenia | 4 | 4 |
| Nausea | 2 | 1 |
| Constipation | 1 | 0 |
Where the frequency of adverse events with nifedipine extended-release tablets and placebo is similar, causal relationship cannot be established.
The following adverse events were reported with an incidence of 3% or less in daily doses up to 90 mg:
Body as a Whole/Systemic: chest pain, leg pain
Central Nervous System: paresthesia, vertigo
Dermatologic: rash
Gastrointestinal: constipation
Musculoskeletal: leg cramps
Respiratory: epistaxis, rhinitis
Urogenital: impotence, urinary frequency
Other adverse events reported with an incidence of less than 1.0% were:
Body as a Whole/Systemic: allergic reaction, asthenia, cellulitis, substernal chest pain, chills, facial edema, lab test abnormal, malaise, neck pain, pelvic pain, pain, photosensitivity reaction
Cardiovascular: atrial fibrillation, bradycardia, cardiac arrest, extrasystole, hypotension, migraine, palpitations, phlebitis, postural hypotension, tachycardia, cutaneous angiectases
Central Nervous System: anxiety, confusion, decreased libido, depression, hypertonia, hypesthesia, insomnia, somnolence
Dermatologic: angioedema, petechial rash, pruritus, sweating
Gastrointestinal: abdominal pain, diarrhea, dry mouth, dysphagia, dyspepsia, eructation, esophagitis, flatulence, gastrointestinal disorder, gastrointestinal hemorrhage, GGT increased, gum disorder, gum hemorrhage, vomiting
Hematologic: eosinophilia, lymphadenopathy
Metabolic: gout, weight loss
Musculoskeletal: arthralgia, arthritis, joint disorder, myalgia, myasthenia
Respiratory: dyspnea, increased cough, rales, pharyngitis, stridor
Special Senses: abnormal vision, amblyopia, conjunctivitis, diplopia, eye disorder, eye hemorrhage, tinnitus
Urogenital/Reproductive: dysuria, kidney calculus, nocturia, breast engorgement, polyuria, urogenital disorder
The following adverse events have been reported rarely in patients given nifedipine in coat core or other formulations: allergenic hepatitis, alopecia, anaphylactic reaction, anemia, arthritis with ANA (+), depression, erythromelalgia, exfoliative dermatitis, fever, gingival hyperplasia, gynecomastia, hyperglycemia, jaundice, leukopenia, mood changes, muscle cramps, nervousness, paranoid syndrome, purpura, shakiness, sleep disturbances, Stevens-Johnson syndrome, syncope, taste perversion, thrombocytopenia, toxic epidermal necrolysis, transient blindness at the peak of plasma level, tremor and urticaria.
OVERDOSAGE
Experience with nifedipine overdosage is limited. Symptoms associated with severe nifedipine overdosage include loss of consciousness, drop in blood pressure, heart rhythm disturbances, metabolic acidosis, hypoxia, cardiogenic shock with pulmonary edema. Generally, overdosage with nifedipine leading to pronounced hypotension calls for active cardiovascular support including monitoring of cardiovascular and respiratory function, elevation of extremities, judicious use of calcium infusion, pressor agents and fluids. Clearance of nifedipine would be expected to be prolonged in patients with impaired liver function. Since nifedipine is highly protein bound, dialysis is not likely to be of any benefit; however, plasmapheresis may be beneficial.
There has been one reported case of massive overdosage with tablets of another extended-release formulation of nifedipine. The main effects of ingestion of approximately 4800 mg of nifedipine in a young man attempting suicide as a result of cocaine-induced depression was initial dizziness, palpitations, flushing, and nervousness.Within several hours of ingestion, nausea, vomiting, and generalized edema developed. No significant hypotension was apparent at presentation, 18 hours post ingestion. Blood chemistry abnormalities consisted of a mild, transient elevation of serum creatinine, and modest elevations of LDH and CPK, but normal SGOT. Vital signs remained stable, no electrocardiographic abnormalities were noted and renal function returned to normal within 24 to 48 hours with routine supportive measures alone. No prolonged sequelae were observed.
The effect of a single 900 mg ingestion of nifedipine capsules in a depressed anginal patient on tricyclic antidepressants was loss of consciousness within 30 minutes of ingestion, and profound hypotension, which responded to calcium infusion, pressor agents, and fluid replacement. A variety of ECG abnormalities were seen in this patient with a history of bundle branch block, including sinus bradycardia and varying degrees of AV block. These dictated the prophylactic placement of a temporary ventricular pacemaker, but otherwise resolved spontaneously. Significant hyperglycemia was seen initially in this patient, but plasma glucose levels rapidly normalized without further treatment.
A young hypertensive patient with advanced renal failure ingested 280 mg of nifedipine capsules at one time, with resulting marked hypotension responding to calcium infusion and fluids. No AV conduction abnormalities, arrhythmias, or pronounced changes in heart rate were noted, nor was there any further deterioration in renal function.
AFEDITAB DOSAGE AND ADMINISTRATION
Dosage should be adjusted according to each patient’s needs. It is recommended that nifedipine extended-release tablets be administered orally once daily on an empty stomach. Afeditab® CR is an extended release dosage form and tablets should be swallowed whole, not bitten or divided. In general, titration should proceed over a 7 to 14 day period starting with 30 mg once daily. Upward titration should be based on therapeutic efficacy and safety. The usual maintenance dose is 30 mg to 60 mg once daily. Titration to doses above 90 mg daily is not recommended.
If discontinuation of Afeditab® CR is necessary, sound clinical practice suggests that the dosage should be decreased gradually with close physician supervision. Co-administration of nifedipine with grapefruit juice is to be avoided (See CLINICAL PHARMACOLOGY and PRECAUTIONS ).
Care should be taken when dispensing Afeditab® CR to assure that the extended-release dosage form has been prescribed.
HOW SUPPLIED
|
Repackaged by Aphena Pharma Solutions - TN. |

|
Afeditab® CR, 30 mg, is available as round, brownish-red, film-coated, unscored tablets, imprinted with ELN 30, and are supplied in bottles of 100 and 500.
Afeditab® CR, 60 mg, is available as round, brownish-red, film-coated, unscored tablets, imprinted with ELN 60, and are supplied in bottles of 100 and 500.
The tablets should be protected from light and moisture and stored below 30°C (86°F). Dispense in tight, light resistant containers as defined in USP/NF.
Distributed by:
Watson Pharma, Inc.
Parsippany, NJ 07054
Manufactured by:
Alkermes Pharma Ireland Limited
Athlone, Co. Westmeath, Ireland
Revised: 09/2011
CP0346E
Repackaging Information
Please reference the How Supplied section listed above for a description of individual tablets or capsules. This drug product has been received by Aphena Pharma - TN in a manufacturer or distributor packaged configuration and repackaged in full compliance with all applicable cGMP regulations. The package configurations available from Aphena are listed below:
| Count | 30mg | 60mg |
| 30 | 43353-792-30 | 43353-782-30 |
| 90 | 43353-792-60 | 43353-782-60 |
| 180 | - | 43353-782-80 |
| 3000 | - | 43353-782-18 |
Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:

Cookeville, TN 38506
20140508SC
PRINCIPAL DISPLAY PANEL - 60mg
NDC 43353-782 - Nifedipine (Afeditab CR®) ER 60mg - Rx Only

PRINCIPAL DISPLAY PANEL - 30mg
NDC 43353-792 - Nifedipine (Afeditab CR®) ER 30mg - Rx Only

AfeditabNifedipine TABLET, FILM COATED, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AfeditabNifedipine TABLET, FILM COATED, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||