Advance White
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients
Sodium fluoride (0.24%)
Purpose
Purpose
Anticavity toothpaste
Uses
Use aids in the prevention of dental decay
Warning
Keep out of reach of children under 6 years of age.
Directions do not swallow supervise children as necessary until capable of using without supervision
adults and children 2 years and older brush teeth throughly after meals or at least twice a day, or use as directed by a dentist or physician
children under 6 years instruct in good brushing and rinsing habits (to minimize swallowing)
children under 2 years consult a dentist or physician
Inactive ingredients Sorbitol, Sodium Bicarbonate (baking soda), Water, Hydrated Silica, Glycerin, Tetrasodium Pyrophosphate, Flavor, Sodium Lauryl Sulfate, Sodium Saccharin, Sodium Lauroyl Sarcosinate, Cellulose Gum, Blue 1, Yellow 5.
Questions or comments? Call 1-800-786-513
5 Monday-Friday 9am-5pm ET or visit www.myoralcare.com
Principal Display Panel
ARM AND HAMMER The Standard of Purity
Fluoride Anti-Cavity Toothpaste TARTAR CONTROL FROSTED MINT FLAVOR
ADVANCE WHITE Gel
Breath Freshening Baking Soda and Frosted Mint
NET WT. 6.0OZ. (170g)
Carton image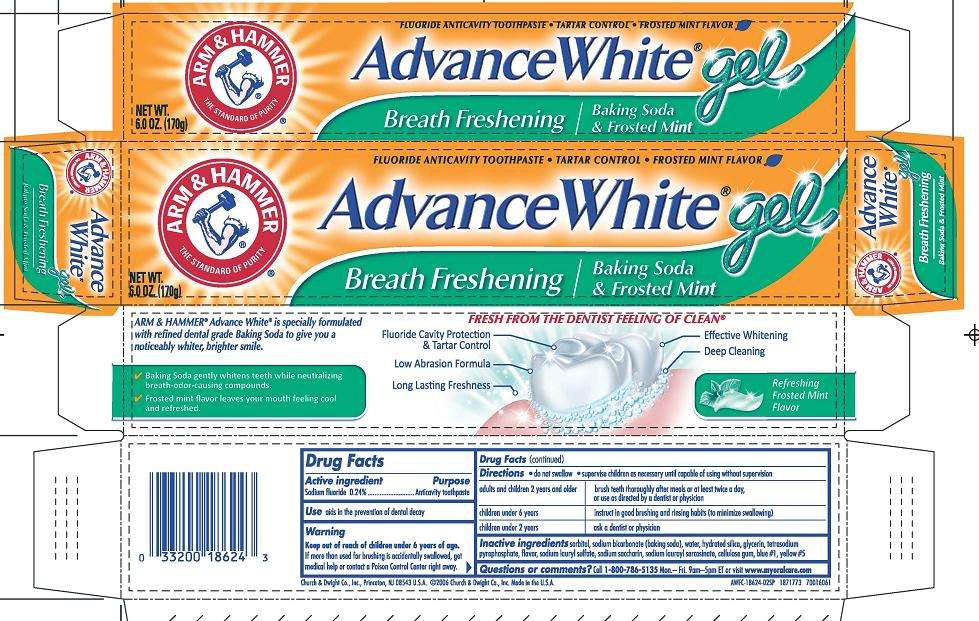
Advance WhiteSodium Fluoride GEL, DENTIFRICE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||