Acyclovir
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACYCLOVIR DESCRIPTION
- VIROLOGY
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- ACYCLOVIR CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- ACYCLOVIR ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ACYCLOVIR DESCRIPTION
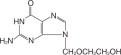
VIROLOGY
Mechanism of Antiviral ActionAntiviral Activities
Drug Resistance
CLINICAL PHARMACOLOGY
PharmacokineticsTable 1.
Table 2. The decrease in bioavailability is a function of the dose and not the dosage form.
Special Populations
Adults with Impaired Renal Function:The half-life and total body clearance of acyclovir are dependent on renal function. A dosage adjustment is recommended for patients with reduced renal function (seeDOSAGE AND ADMINISTRATION).
Geriatrics:Acyclovir plasma concentrations are higher in geriatric patients compared to younger adults, in part due to age-related changes in renal function. Dosage reduction may be required in geriatric patients with underlying renal impairment (seePRECAUTIONS: Geriatric Use).
Pediatrics:In general, the pharmacokinetics of acyclovir in pediatric patients is similar to that of adults. Mean half-life after oral doses of 300 mg/m2 and 600 mg/m2 in pediatric patients aged 7 months to 7 years was 2.6 hours (range 1.59 to 3.74 hours).
Drug Interactions
Clinical Trials
Initial Genital Herpes:Double-blind, placebo-controlled studies have demonstrated that orally administered acyclovir significantly reduced the duration of acute infection and duration of lesion healing. The duration of pain and new lesion formation was decreased in some patient groups.
Recurrent Genital Herpes:Double-blind, placebo-controlled studies in patients with frequent recurrences (6 or more episodes per year) have shown that orally administered acyclovir given daily for 4 months to 10 years prevented or reduced the frequency and/or severity of recurrences in greater than 95% of patients.
Herpes Zoster Infections:In a double-blind, placebo-controlled study of immunocompetent patients with localized cutaneous zoster infection, acyclovir (800 mg 5 times daily for 10 days) shortened the times to lesion scabbing, healing, and complete cessation of pain, and reduced the duration of viral shedding and the duration of new lesion formation.
Chickenpox:Three randomized, double-blind, placebo-controlled trials were conducted in 993 pediatric patients aged 2 to 18 years with chickenpox. All patients were treated within 24 hours after the onset of rash. In 2 trials, acyclovir was administered at 20 mg/kg 4 times daily (up to 3,200 mg per day) for 5 days. In the third trial, doses of 10, 15, or 20 mg/kg were administered 4 times daily for 5 to 7 days.
INDICATIONS & USAGE
Herpes Zoster InfectionsGenital Herpes
Chickenpox
ACYCLOVIR CONTRAINDICATIONS
WARNINGS
ADVERSE REACTIONS: Observed During Clinical PracticeandOVERDOSAGE). Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), which has resulted in death, has occurred in immunocompromised patients receiving acyclovir therapy.PRECAUTIONS
DOSAGE AND ADMINISTRATION). Caution should also be exercised when administering acyclovir to patients receiving potentially nephrotoxic agents since this may increase the risk of renal dysfunction and/or the risk of reversible central nervous system symptoms such as those that have been reported in patients treated with intravenous acyclovir. Adequate hydration should be maintained.INFORMATION FOR PATIENTS
Herpes Zoster:There are no data on treatment initiated more than 72 hours after onset of the zoster rash. Patients should be advised to initiate treatment as soon as possible after a diagnosis of herpes zoster.
Genital Herpes Infections:Patients should be informed that acyclovir is not a cure for genital herpes. There are no data evaluating whether acyclovir will prevent transmission of infection to others. Because genital herpes is a sexually transmitted disease, patients should avoid contact with lesions or intercourse when lesions and/or symptoms are present to avoid infecting partners. Genital herpes can also be transmitted in the absence of symptoms through asymptomatic viral shedding. If medical management of a genital herpes recurrence is indicated, patients should be advised to initiate therapy at the first sign or symptom of an episode.
Chickenpox:Chickenpox in otherwise healthy children is usually a self-limited disease of mild to moderate severity. Adolescents and adults tend to have more severe disease. Treatment was initiated within 24 hours of the typical chickenpox rash in the controlled studies, and there is no information regarding the effects of treatment begun later in the disease course.
DRUG INTERACTIONS
CLINICAL PHARMACOLOGY: Pharmacokinetics.CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CLINICAL PHARMACOLOGY: Pharmacokinetics).PREGNANCY
Teratogenic Effects:Pregnancy Category B. Acyclovir administered during organogenesis was not teratogenic in the mouse (450 mg/kg/day, p.o.), rabbit (50 mg/kg/day, s.c. and IV), or rat (50 mg/kg/day, s.c.). These exposures resulted in plasma levels 9 and 18, 16 and 106, and 11 and 22 times, respectively, human levels.NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
CLINICAL PHARMACOLOGY,ADVERSE REACTIONS: Observed During Clinical Practice,andDOSAGE AND ADMINISTRATION).ACYCLOVIR ADVERSE REACTIONS
Herpes SimplexShort-Term Administration:The most frequent adverse events reported during clinical trials of treatment of genital herpes with acyclovir 200 mg administered orally 5 times daily every 4 hours for 10 days were nausea and/or vomiting in 8 of 298 patient treatments (2.7%). Nausea and/or vomiting occurred in 2 of 287 (0.7%) patients who received placebo.
Long-Term Administration:The most frequent adverse events reported in a clinical trial for the prevention of recurrences with continuous administration of 400 mg (two 200-mg capsules) 2 times daily for 1 year in 586 patients treated with acyclovir were nausea (4.8%) and diarrhea (2.4%). The 589 control patients receiving intermittent treatment of recurrences with acyclovir for 1 year reported diarrhea (2.7%), nausea (2.4%), and headache (2.2%).
Herpes Zoster
Chickenpox
Observed During Clinical Practice
General:Anaphylaxis, angioedema, fever, headache, pain, peripheral edema.
Nervous:Aggressive behavior, agitation, ataxia, coma, confusion, decreased consciousness, delirium, dizziness, dysarthria, encephalopathy, hallucinations, paresthesia, psychosis, seizure, somnolence, tremors. These symptoms may be marked, particularly in older adults or in patients with renal impairment (seePRECAUTIONS).
Digestive:Diarrhea, gastrointestinal distress, nausea.
Hematologic and Lymphatic:Anemia, leukocytoclastic vasculitis, leukopenia, lymphadenopathy, thrombocytopenia.
Hepatobiliary Tract and Pancreas:Elevated liver function tests, hepatitis, hyperbilirubinemia, jaundice.
Musculoskeletal:Myalgia.
Skin:Alopecia, erythema multiforme, photosensitive rash, pruritus, rash, Stevens-Johnson syndrome, toxic epidermal necrolysis, urticaria.
Special Senses:Visual abnormalities.
Urogenital:Renal failure, renal pain (may be associated with renal failure), elevated blood urea nitrogen, elevated creatinine, hematuria (seeWARNINGS).
OVERDOSAGE
DOSAGE AND ADMINISTRATION).DOSAGE & ADMINISTRATION
Acute Treatment of Herpes ZosterGenital Herpes
Treatment of Initial Genital Herpes:200 mg every 4 hours, 5 times daily for 10 days.
Chronic Suppressive Therapy for Recurrent Disease:
Intermittent Therapy:200 mg every 4 hours, 5 times daily for 5 days. Therapy should be initiated at the earliest sign or symptom (prodrome) of recurrence.
Treatment of Chickenpox
Children (2 years of age and older):20 mg/kg per dose orally 4 times daily (80 mg/kg/day) for 5 days. Children over 40 kg should receive the adult dose for chickenpox.
Adults and Children over 40 kg:800 mg 4 times daily for 5 days.
Patients With Acute or Chronic Renal Impairment
Table 3:
Hemodialysis
Peritoneal Dialysis
Bioequivalence of Dosage Forms
HOW SUPPLIED


STORAGE AND HANDLING
Store at 20 to 25 C (68 to 77 F). [See USP Controlled Room Temperature.]Protect from moisture. Keep this and all medication out of the reach of children.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

AcyclovirAcyclovir TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!