Acitretin
ACITRETIN CAPSULES USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACITRETIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- ACITRETIN INDICATIONS AND USAGE
- ACITRETIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ACITRETIN ADVERSE REACTIONS
- OVERDOSAGE
- ACITRETIN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- MEDICATION GUIDE
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION

1135
1138
1136
Rx only
CONTRAINDICATIONS AND WARNINGS: Pregnancy
Acitretin must not be used by females who are pregnant, or who intend to become pregnant during therapy or at any time for at least 3 years following discontinuation of therapy. Acitretin also must not be used by females who may not use reliable contraception while undergoing treatment and for at least 3 years following discontinuation of treatment. Acitretin is a metabolite of etretinate (Tegison®), and major human fetal abnormalities have been reported with the administration of acitretin and etretinate. Potentially, any fetus exposed can be affected.
Clinical evidence has shown that concurrent ingestion of acitretin and ethanol has been associated with the formation of etretinate, which has a significantly longer elimination half-life than acitretin. Because the longer elimination half-life of etretinate would increase the duration of teratogenic potential for female patients, ethanol must not be ingested by female patients either during treatment with acitretin or for 2 months after cessation of therapy. This allows for elimination of acitretin, thus removing the substrate for transesterification to etretinate. The mechanism of the metabolic process for conversion of acitretin to etretinate has not been fully defined. It is not known whether substances other than ethanol are associated with transesterification.
Acitretin has been shown to be embryotoxic and/or teratogenic in rabbits, mice, and rats at oral doses of 0.6, 3, and 15 mg/kg, respectively. These doses are approximately 0.2, 0.3, and 3 times the maximum recommended therapeutic dose, respectively, based on a mg/m2 comparison.
Major human fetal abnormalities associated with acitretin and/or etretinate administration have been reported including meningomyelocele; meningoencephalocele, multiple synostoses; facial dysmorphia; syndactyly; absence of terminal phalanges; malformations of hip, ankle, and forearm; low-set ears; high palate; decreased cranial volume; cardiovascular malformation; and alterations of the skull and cervical vertebrae.
Acitretin should be prescribed only by those who have special competence in the diagnosis and treatment of severe psoriasis, are experienced in the use of systemic retinoids, and understand the risk of teratogenicity.
Because of the teratogenicity of acitretin, a program called the T.A.P.P. program, Take A ction to Prevent Pregnancy, has been developed to educate women of childbearing potential and their healthcare providers about the serious risks associated with acitretin and to help prevent pregnancies from occurring with the use of this drug and for 3 years after its discontinuation. The T.A.P.P. program requirements are described below and program materials are available at http://www.tevagenerics.com/acitretin or may be requested by calling 1-855-850-2138 (see also PRECAUTIONS section).
Important Information for Women of Childbearing Potential
Acitretin should be considered only for women with severe psoriasis unresponsive to other therapies or whose clinical condition contraindicates the use of other treatments.
Females of reproductive potential must not be given a prescription for acitretin until pregnancy is excluded. Acitretin is contraindicated in females of reproductive potential unless the patient meets ALL of the following conditions:
- Must have had 2 negative urine or serum pregnancy tests with a sensitivity of at least 25 mIU/mL before receiving the initial prescription for acitretin. The first test (a screening test) is obtained by the prescriber when the decision is made to pursue therapy with acitretin. The second pregnancy test (a confirmation test) should be done during the first 5 days of the menstrual period immediately preceding the beginning of therapy with acitretin. For patients with amenorrhea, the second test should be done at least 11 days after the last act of unprotected sexual intercourse (without using 2 effective forms of contraception [birth control] simultaneously).
- Must have a pregnancy test repeated every month during treatment with acitretin. The patient must have a negative result from a urine or serum pregnancy test before receiving a prescription for acitretin. To encourage compliance with this recommendation, a limited supply of the drug should be prescribed. For at least 3 years after discontinuing therapy with acitretin, a pregnancy test must be repeated every 3 months.
- Must have selected and have committed to use 2 effective forms of contraception (birth control) simultaneously, at least 1 of which must be a primary form, unless absolute abstinence is the chosen method, or the patient has undergone a hysterectomy or is clearly postmenopausal.
- Patients must use 2 effective forms of contraception (birth control) simultaneously for at least 1 month prior to initiation of therapy with acitretin, during therapy with acitretin, and for at least 3 years after discontinuing therapy with acitretin. An Acitretin Referral Form is available so that patients can receive an initial free contraceptive counseling session and pregnancy testing. Counseling about contraception and behaviors associated with an increased risk of pregnancy must be repeated on a monthly basis by the prescriber during therapy with acitretin and every 3 months for at least 3 years following discontinuation of acitretin.
Effective forms of contraception include both primary and secondary forms of contraception. Primary forms of contraception include: tubal ligation, partner’s vasectomy, intrauterine devices, birth control pills, and injectable/implantable/ insertable/topical hormonal birth control products. Secondary forms of contraception include latex condoms (with or without spermicide), diaphragms and cervical caps (which must be used with a spermicide).
Any birth control method can fail. Therefore, it is critically important that women of childbearing potential use 2 effective forms of contraception (birth control) simultaneously. It has not been established if there is a pharmacokinetic interaction between acitretin and combined oral contraceptives. However, it has been established that acitretin interferes with the contraceptive effect of microdosed progestin preparations.1 Microdosed “minipill” progestin preparations are not recommended for use with acitretin. It is not known whether other progestational contraceptives, such as implants and injectables, are adequate methods of contraception during acitretin therapy.
Prescribers are advised to consult the package insert of any medication administered concomitantly with hormonal contraceptives, since some medications may decrease the effectiveness of these birth control products. Patients should be prospectively cautioned not to self-medicate with the herbal supplement St. John’s wort because a possible interaction has been suggested with hormonal contraceptives based on reports of breakthrough bleeding on oral contraceptives shortly after starting St. John’s wort. Pregnancies have been reported by users of combined hormonal contraceptives who also used some form of St. John’s wort (see PRECAUTIONS).
- Must have signed a Patient Agreement/Informed Consent for Female Patients that contains warnings about the risk of potential birth defects if the fetus is exposed to acitretin, about contraceptive failure, about the fact that they must not ingest beverages or products containing ethanol while taking acitretin and for 2 months after acitretin treatment has been discontinued, and about preventing pregnancy while taking acitretin and for at least 3 years after discontinuing acitretin.
If pregnancy does occur during therapy with acitretin or at any time for at least 3 years following discontinuation of acitretin, the prescriber and patient should discuss the possible effects on the pregnancy. The available information is as follows:
Acitretin, the active metabolite of etretinate, is teratogenic and is contraindicated during pregnancy. The risk of severe fetal malformations is well established when systemic retinoids are taken during pregnancy. Pregnancy must also be prevented after stopping acitretin therapy, while the drug is being eliminated to below a threshold blood concentration that would be associated with an increased incidence of birth defects. Because this threshold has not been established for acitretin in humans and because elimination rates vary among patients, the duration of posttherapy contraception to achieve adequate elimination cannot be calculated precisely. It is strongly recommended that contraception be continued for at least 3 years after stopping treatment with acitretin, based on the following considerations:
- In the absence of transesterification to form etretinate, greater than 98% of the acitretin would be eliminated within 2 months, assuming a mean elimination half-life of 49 hours.
-
In cases where etretinate is formed, as has been demonstrated with concomitant administration of acitretin and ethanol,
♦ greater than 98% of the etretinate formed would be eliminated in 2 years, assuming a mean elimination half-life of 120 days.
♦ greater than 98% of the etretinate formed would be eliminated in 3 years, based on the longest demonstrated elimination half-life of 168 days.
However, etretinate was found in plasma and subcutaneous fat in one patient reported to have had sporadic alcohol intake, 52 months after she stopped acitretin therapy.2
-
Severe birth defects have been reported where conception occurred during the time interval when the patient was being treated with acitretin and/or etretinate. In addition, severe birth defects have also been reported when conception occurred after the mother completed therapy. These cases have been reported both prospectively (before the outcome was known) and retrospectively (after the outcome was known). The events below are listed without distinction as to whether the reported birth defects are consistent with retinoid-induced embryopathy or not.
♦ There have been 318 prospectively reported cases involving pregnancies and the use of etretinate, acitretin, or both. In 238 of these cases, the conception occurred after the last dose of etretinate (103 cases), acitretin (126), or both (9). Fetal outcome remained unknown in approximately one-half of these cases, of which 62 were terminated and 14 were spontaneous abortions. Fetal outcome is known for the other 118 cases and 15 of the outcomes were abnormal (including cases of absent hand/wrist, clubfoot, GI malformation, hypocalcemia, hypotonia, limb malformation, neonatal apnea/anemia, neonatal ichthyosis, placental disorder/death, undescended testicle, and 5 cases of premature birth). In the 126 prospectively reported cases where conception occurred after the last dose of acitretin only, 43 cases involved conception at least 1 year but less than 2 years after the last dose. There were 3 reports of abnormal outcomes out of these 43 cases (involving limb malformation, GI tract malformations, and premature birth). There were only 4 cases where conception occurred at least 2 years after the last dose but there were no reports of birth defects in these cases.
♦ There is also a total of 35 retrospectively reported cases where conception occurred at least 1 year after the last dose of etretinate, acitretin, or both. From these cases there are 3 reports of birth defects when the conception occurred at least 1 year but less than 2 years after the last dose of acitretin (including heart malformations, Turner’s Syndrome, and unspecified congenital malformations) and 4 reports of birth defects when conception occurred 2 or more years after the last dose of acitretin (including foot malformation, cardiac malformations [2 cases], and unspecified neonatal and infancy disorder). There were 3 additional abnormal outcomes in cases where conception occurred 2 or more years after the last dose of etretinate (including chromosome disorder, forearm aplasia, and stillbirth).
♦ Females who have taken Tegison (etretinate) must continue to follow the contraceptive recommendations for Tegison. Tegison is no longer marketed in the U.S.; for information, call 1-888-838-2872.
♦ Patients should not donate blood during and for at least 3 years following the completion of therapy with acitretin because women of childbearing potential must not receive blood from patients being treated with acitretin.
Important Information For Males Taking Acitretin
-
Patients should not donate blood during and for at least 3 years following therapy with acitretin because women of childbearing potential must not receive blood from patients being treated with acitretin.
- Samples of seminal fluid from 3 male patients treated with acitretin and 6 male patients treated with etretinate have been assayed for the presence of acitretin. The maximum concentration of acitretin observed in the seminal fluid of these men was 12.5 ng/mL. Assuming an ejaculate volume of 10 mL, the amount of drug transferred in semen would be 125 ng, which is 1/200,000 of a single 25 mg capsule. Thus, although it appears that residual acitretin in seminal fluid poses little, if any, risk to a fetus while a male patient is taking the drug or after it is discontinued, the no-effect limit for teratogenicity is unknown and there is no registry for birth defects associated with acitretin. The available data are as follows:
There have been 25 cases of reported conception when the male partner was taking acitretin. The pregnancy outcome is known in 13 of these 25 cases. Of these, 9 reports were retrospective and 4 were prospective (meaning the pregnancy was reported prior to knowledge of the outcome)3.
| Timing of Paternal Acitretin Treatment Relative to Conception |
Delivery of Healthy Neonate |
Spontaneous Abortion |
Induced Abortion |
Total |
| At time of conception | 5 |
5 | 1 | 11 |
| Discontinued ~4 weeks prior | 0 | 0 | 1 |
1 |
| Discontinued ~6 to 8 months prior | 0 | 1 | 0 | 1 |
For All Patients: AN ACITRETIN MEDICATION GUIDE MUST BE GIVEN TO THE PATIENT EACH TIME ACITRETIN IS DISPENSED, AS REQUIRED BY LAW.
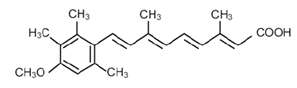
ACITRETIN DESCRIPTION
Acitretin, USP (micronized), a retinoid, is available in 10 mg, 17.5 mg, and 25 mg gelatin capsules for oral administration. Chemically, acitretin is all-trans-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoic acid. It is a metabolite of etretinate and is related to both retinoic acid and retinol (vitamin A). It is a yellow to greenish-yellow crystalline powder. The structural formula is:
Each capsule contains acitretin, USP (micronized) 10 mg, 17.5 mg, and 25 mg. Inactive ingredients are crospovidone, microcrystalline cellulose, poloxamer, povidone, sodium ascorbate and sodium lauryl sulfate.
The 10 mg, 17.5, and 25 mg gelatin capsule shells contain gelatin and titanium dioxide. The 10 mg and 25 mg capsule shells also contain D&C yellow no. 10, FD&C blue no. 1, and FD&C red no. 40. The 17.5 mg capsule shells also contain red iron oxide and yellow iron oxide. The 25 mg gelatin capsule shells also contain FD&C yellow no. 6. The edible imprinting ink contains black iron oxide, D&C yellow no. 10 aluminum lake, FD&C blue no. 1 aluminum lake, FD&C blue no. 2 aluminum lake, FD&C red no. 40 aluminum lake, propylene glycol and shellac glaze.
Meets USP Dissolution Test 2.
CLINICAL PHARMACOLOGY
The mechanism of action of acitretin is unknown.
Pharmacokinetics
Absorption
Oral absorption of acitretin is optimal when given with food. For this reason, acitretin was given with food in all of the following trials. After administration of a single 50 mg oral dose of acitretin to 18 healthy subjects, maximum plasma concentrations ranged from 196 to 728 ng/mL (mean: 416 ng/mL) and were achieved in 2 to 5 hours (mean: 2.7 hours). The oral absorption of acitretin is linear and proportional with increasing doses from 25 to 100 mg. Approximately 72% (range: 47% to 109%) of the administered dose was absorbed after a single 50 mg dose of acitretin was given to 12 healthy subjects.
Distribution
Acitretin is more than 99.9% bound to plasma proteins, primarily albumin.
Metabolism (See Pharmacokinetic Drug Interactions , Ethanol.)
Following oral absorption, acitretin undergoes extensive metabolism and interconversion by simple isomerization to its 13-cis form (cis-acitretin). The formation of cis-acitretin relative to parent compound is not altered by dose or fed/fast conditions of oral administration of acitretin. Both parent compound and isomer are further metabolized into chain-shortened breakdown products and conjugates, which are excreted. Following multiple-dose administration of acitretin, steady-state concentrations of acitretin and cis-acitretin in plasma are achieved within approximately 3 weeks.
Elimination
The chain-shortened metabolites and conjugates of acitretin and cis-acitretin are ultimately excreted in the feces (34% to 54%) and urine (16% to 53%). The terminal elimination half-life of acitretin following multiple-dose administration is 49 hours (range: 33 to 96 hours), and that of cis-acitretin under the same conditions is 63 hours (range: 28 to 157 hours). The accumulation ratio of the parent compound is 1.2; that of cis-acitretin is 6.6.
Special Populations
Psoriasis
In an 8 week trial of acitretin pharmacokinetics in subjects with psoriasis, mean steady-state trough concentrations of acitretin increased in a dose-proportional manner with dosages ranging from 10 to 50 mg daily. Acitretin plasma concentrations were nonmeasurable (< 4 ng/mL) in all subjects 3 weeks after cessation of therapy.
Elderly
In a multiple-dose trial in healthy young (n = 6) and elderly (n = 8) subjects, a 2 fold increase in acitretin plasma concentrations were seen in elderly subjects, although the elimination half-life did not change.
Renal Failure
Plasma concentrations of acitretin were significantly (59.3%) lower in subjects with end-stage renal failure (n = 6) when compared with age-matched controls, following single 50 mg oral doses. Acitretin was not removed by hemodialysis in these subjects.
Pharmacokinetic Drug Interactions
(See also boxed CONTRAINDICATIONS AND WARNINGS and PRECAUTIONS, Drug Interactions )
In studies of in vivo pharmacokinetic drug interactions, no interaction was seen between acitretin and cimetidine, digoxin, phenprocoumon, or glyburide.
Ethanol
Clinical evidence has shown that etretinate (a retinoid with a much longer half-life, see below) can be formed with concurrent ingestion of acitretin and ethanol. In a 2 way crossover trial, all 10 subjects formed etretinate with concurrent ingestion of a single 100 mg oral dose of acitretin during a 3 hour period of ethanol ingestion (total ethanol, approximately 1.4 g/kg body weight). A mean peak etretinate concentration of 59 ng/mL (range: 22 to 105 ng/mL) was observed, and extrapolation of AUC values indicated that the formation of etretinate in this trial was comparable to a single 5 mg oral dose of etretinate. There was no detectable formation of etretinate when a single 100 mg oral dose of acitretin was administered without concurrent ethanol ingestion, although the formation of etretinate without concurrent ethanol ingestion cannot be excluded (see boxed CONTRAINDICATIONS AND WARNINGS ). Of 93 evaluable psoriatic subjects on acitretin therapy in several foreign trials (10 to 80 mg/day), 16% had measurable etretinate levels (> 5 ng/mL).
Etretinate has a much longer elimination half-life compared with that of acitretin. In one trial the apparent mean terminal half-life after 6 months of therapy was approximately 120 days (range: 84 to 168 days). In another trial of 47 subjects treated chronically with etretinate, 5 had detectable serum drug levels (in the range of 0.5 to 12 ng/mL) 2.1 to 2.9 years after therapy was discontinued. The long half-life appears to be due to storage of etretinate in adipose tissue.
Progestin-Only Contraceptives
It has not been established if there is a pharmacokinetic interaction between acitretin and combined oral contraceptives. However, it has been established that acitretin interferes with the contraceptive effect of microdosed progestin preparations.1 Microdosed “minipill” progestin preparations are not recommended for use with acitretin. It is not known whether other progestational contraceptives, such as implants and injectables, are adequate methods of contraception during acitretin therapy.
CLINICAL STUDIES
In 2 double-blind placebo-controlled trials, acitretin was administered once daily to subjects with severe psoriasis (e.g., covering at least 10% to 20% of the body surface area). At 8 weeks (see Table 1 ) subjects treated in Trial A with 50 mg of acitretin per day showed significant improvements (P ≤ 0.05) relative to baseline and to placebo in the physician’s global evaluation and in the mean ratings of severity of psoriasis (scaling, thickness, and erythema). In Trial B, differences from baseline and from placebo were statistically significant (P ≤ 0.05) for all variables at both the 25 mg and 50 mg doses; it should be noted for Trial B that no statistical adjustment for multiplicity was carried out.
|
Efficacy |
Trial A | Trial B | |||
| Total daily dose | Total daily dose | ||||
|
Placebo (N = 29) |
50 mg (N = 29) |
Placebo (N = 72) |
25 mg (N = 74) |
50 mg (N = 71) |
|
| Physician’s Global Evaluation | |||||
| Baseline | 4.62 | 4.55 | 4.43 | 4.37 | 4.49 |
| Mean Change After 8 Weeks | −0.29 | −2 |
−0.06 | −1.06 |
−1.57 |
| Scaling | |||||
| Baseline | 4.1 | 3.76 | 3.97 | 4.11 | 4.1 |
| Mean Change After 8 Weeks | −0.22 | −1.62 |
−0.21 | −1.50 |
−1.78 |
| Thickness | |||||
| Baseline | 4.1 | 4.1 | 4.03 | 4.11 | 4.2 |
| Mean Change After 8 Weeks | −0.39 | −2.1 |
−0.18 | −1.43 |
−2.11 |
| Erythema | |||||
| Baseline | 4.21 | 4.59 | 4.42 | 4.24 | 4.45 |
| Mean Change After 8 Weeks | −0.33 | −2.1 |
−0.37 | −1.12 |
−1.65 |
The efficacy variables consisted of: the mean severity rating of scale, lesion thickness, erythema, and the physician’s global evaluation of the current status of the disease. Ratings of scaling, erythema, and lesion thickness, and the ratings of the global assessments were made using a 7 point scale (0 = none, 1 = trace, 2 = mild, 3 = mild-moderate, 4 = moderate, 5 = moderate-severe, 6 = severe).
A subset of 141 subjects from both pivotal Trials A and B continued to receive acitretin in an open fashion for up to 24 weeks. At the end of the treatment period, all efficacy variables, as indicated in Table 2, were significantly improved (P ≤ 0.01) from baseline, including extent of psoriasis, mean ratings of psoriasis severity, and physician’s global evaluation.
| Variables | Trial A | Trial B |
| Mean Total Daily Acitretin Dose (mg) | 42.8 | 43.1 |
| Mean Duration of Therapy (Weeks) | 21.1 | 22.6 |
| Physician’s Global Evaluation | N = 39 | N = 98 |
| Baseline | 4.51 | 4.43 |
| Mean Change From Baseline | −2.26 |
−2.6 |
| Scaling | N = 59 | N = 132 |
| Baseline | 3.97 | 4.07 |
| Mean Change From Baseline | −2.15 |
−2.42 |
| Thickness | N = 59 | N = 132 |
| Baseline | 4 | 4.12 |
| Mean Change From Baseline | −2.44 |
−2.66 |
| Erythema | N = 59 | N = 132 |
| Baseline | 4.35 | 4.33 |
| Mean Change From Baseline | −2.31 |
−2.29 |
The efficacy variables consisted of: the mean severity rating of scale, lesion thickness, erythema, and the physician’s global evaluation of the current status of the disease. Ratings of scaling, erythema, and lesion thickness, and the ratings of the global assessments were made using a 7 point scale (0 = none, 1 = trace, 2 = mild, 3 = mild-moderate, 4 = moderate, 5 = moderate-severe, 6 = severe).
All efficacy variables improved significantly in a subset of 55 subjects from Trial A treated for a second, 6 month maintenance course of therapy (for a total of 12 months of treatment); a small subset of subjects (n = 4) from Trial A continued to improve after a third 6 month course of therapy (for a total of 18 months of treatment).
ACITRETIN INDICATIONS AND USAGE
Acitretin Capsules USP are indicated for the treatment of severe psoriasis in adults. Because of significant adverse effects associated with their use, Acitretin Capsules USP should be prescribed only by those knowledgeable in the systemic use of retinoids. In females of reproductive potential, Acitretin Capsules USP should be reserved for non-pregnant patients who are unresponsive to other therapies or whose clinical condition contraindicates the use of other treatments (see boxed CONTRAINDICATIONS AND WARNINGS — Acitretin Capsules USP can cause severe birth defects).
Most patients experience relapse of psoriasis after discontinuing therapy. Subsequent courses, when clinically indicated, have produced efficacy results similar to the initial course of therapy.
ACITRETIN CONTRAINDICATIONS
Pregnancy Category X
(See boxed CONTRAINDICATIONS AND WARNINGS .)
Acitretin is contraindicated in patients with severely impaired liver or kidney function and in patients with chronic abnormally elevated blood lipid values (see boxed WARNINGS, Hepatotoxicity; WARNINGS, Lipids and Possible Cardiovascular Effects ; and PRECAUTIONS ).
An increased risk of hepatitis has been reported to result from combined use of methotrexate and etretinate. Consequently, the combination of methotrexate with acitretin is also contraindicated (see PRECAUTIONS, Drug Interactions ).
Since both acitretin and tetracyclines can cause increased intracranial pressure, their combined use is contraindicated (see WARNINGS, Pseudotumor Cerebri ).
Acitretin is contraindicated in cases of hypersensitivity to the preparation (acitretin or excipients) or to other retinoids.
WARNINGS
(See also boxed CONTRAINDICATIONS AND WARNINGS .)
Hepatotoxicity
Of the 525 subjects treated in U.S. clinical trials, 2 had clinical jaundice with elevated serum bilirubin and transaminases considered related to treatment with acitretin. Liver function test results in these subjects returned to normal after acitretin was discontinued. Two of the 1,289 subjects treated in European clinical trials developed biopsy-confirmed toxic hepatitis. A second biopsy in one of these subjects revealed nodule formation suggestive of cirrhosis. One subject in a Canadian clinical trial of 63 subjects developed a 3 fold increase of transaminases. A liver biopsy of this subject showed mild lobular disarray, multifocal hepatocyte loss, and mild triaditis of the portal tracts compatible with acute reversible hepatic injury. The subject’s transaminase levels returned to normal 2 months after acitretin was discontinued.
The potential of therapy with acitretin to induce hepatotoxicity was prospectively evaluated using liver biopsies in an open-label trial of 128 subjects. Pretreatment and posttreatment biopsies were available for 87 subjects. A comparison of liver biopsy findings before and after therapy revealed 49 (58%) subjects showed no change, 21 (25%) improved, and 14 (17%) subjects had a worsening of their liver biopsy status. For 6 subjects, the classification changed from class 0 (no pathology) to class I (normal fatty infiltration; nuclear variability and portal inflammation; both mild); for 7 subjects, the change was from class I to class II (fatty infiltration, nuclear variability, portal inflammation, and focal necrosis; all moderate to severe); and for 1 subject, the change was from class II to class IIIb (fibrosis, moderate to severe). No correlation could be found between liver function test result abnormalities and the change in liver biopsy status, and no cumulative dose relationship was found.
Elevations of AST (SGOT), ALT (SGPT), GGT (GGTP), or LDH have occurred in approximately 1 in 3 subjects treated with acitretin. Of the 525 subjects treated in clinical trials in the U.S., treatment was discontinued in 20 (3.8%) due to elevated liver function test results. If hepatotoxicity is suspected during treatment with acitretin, the drug should be discontinued and the etiology further investigated.
Ten of 652 subjects treated in U.S. clinical trials of etretinate, of which acitretin is the active metabolite, had clinical or histologic hepatitis considered to be possibly or probably related to etretinate treatment.
There have been reports of hepatitis-related deaths worldwide; a few of these subjects had received etretinate for a month or less before presenting with hepatic symptoms or signs.
Hyperostosis
In adults receiving long-term treatment with acitretin, appropriate examinations should be periodically performed in view of possible ossification abnormalities (see ADVERSE REACTIONS ). Because the frequency and severity of iatrogenic bony abnormality in adults is low, periodic radiography is only warranted in the presence of symptoms or long-term use of acitretin. If such disorders arise, the continuation of therapy should be discussed with the patient on the basis of a careful risk/benefit analysis. In clinical trials with acitretin, subjects were prospectively evaluated for evidence of development or change in bony abnormalities of the vertebral column, knees, and ankles.
Vertebral Results
Of 380 subjects treated with acitretin, 15% had preexisting abnormalities of the spine which showed new changes or progression of preexisting findings. Changes included degenerative spurs, anterior bridging of spinal vertebrae, diffuse idiopathic skeletal hyperostosis, ligament calcification, and narrowing and destruction of a cervical disc space. De novo changes (formation of small spurs) were seen in 3 subjects after 1½ to 2½ years.
Skeletal Appendicular Results
Six of 128 patients treated with acitretin showed abnormalities in the knees and ankles before treatment that progressed during treatment. In 5, these changes involved the formation of additional spurs or enlargement of existing spurs. The sixth subject had degenerative joint disease which worsened. No subjects developed spurs de novo. Clinical complaints did not predict radiographic changes.
Lipids and Possible Cardiovascular Effects
Blood lipid determinations should be performed before acitretin is administered and again at intervals of 1 to 2 weeks until the lipid response to the drug is established, usually within 4 to 8 weeks. In subjects receiving acitretin during clinical trials, 66% and 33% experienced elevation in triglycerides and cholesterol, respectively. Decreased high density lipoproteins (HDL) occurred in 40% of subjects. These effects of acitretin were generally reversible upon cessation of therapy.
Subjects with an increased tendency to develop hypertriglyceridemia included those with disturbances of lipid metabolism, diabetes mellitus, obesity, increased alcohol intake, or a familial history of these conditions. Because of the risk of hypertriglyceridemia, serum lipids must be more closely monitored in high-risk patients and during long-term treatment.
Hypertriglyceridemia and lowered HDL may increase a patient’s cardiovascular risk status. Although no causal relationship has been established, there have been postmarketing reports of acute myocardial infarction or thromboembolic events in patients on therapy with acitretin. In addition, elevation of serum triglycerides to greater than 800 mg/dL has been associated with fatal fulminant pancreatitis. Therefore, dietary modifications, reduction in dose of acitretin, or drug therapy should be employed to control significant elevations of triglycerides. If, despite these measures, hypertriglyceridemia and low HDL levels persist, the discontinuation of acitretin should be considered.
Ophthalmologic Effects
The eyes and vision of 329 subjects treated with acitretin were examined by ophthalmologists. The findings included dry eyes (23%), irritation of eyes (9%), and brow and lash loss (5%). The following were reported in less than 5% of patients: Bell’s Palsy, blepharitis and/or crusting of lids, blurred vision, conjunctivitis, corneal epithelial abnormality, cortical cataract, decreased night vision, diplopia, itchy eyes or eyelids, nuclear cataract, pannus, papilledema, photophobia, posterior subcapsular cataract, recurrent sties, and subepithelial corneal lesions.
Any patient treated with acitretin who is experiencing visual difficulties should discontinue the drug and undergo ophthalmologic evaluation.
Pancreatitis
Lipid elevations occur in 25% to 50% of patients treated with acitretin. Triglyceride increases sufficient to be associated with pancreatitis are much less common, although fatal fulminant pancreatitis has been reported. There have been rare reports of pancreatitis during therapy with acitretin in the absence of hypertriglyceridemia.
Pseudotumor Cerebri
Acitretin and other retinoids administered orally have been associated with cases of pseudotumor cerebri (benign intracranial hypertension). Some of these events involved concomitant use of isotretinoin and tetracyclines. However, the event seen in a single patient receiving acitretin was not associated with tetracycline use. Early signs and symptoms include papilledema, headache, nausea and vomiting, and visual disturbances. Patients with these signs and symptoms should be examined for papilledema and, if present, should discontinue acitretin immediately and be referred for neurological evaluation and care. Since both acitretin and tetracyclines can cause increased intracranial pressure, their combined use is contraindicated (see CONTRAINDICATIONS ).
PRECAUTIONS
A description of the T.A.P.P. materials is provided below. The main goals of the materials are to explain the program requirements, to reinforce the educational messages, and to assess program effectiveness.
The T.A.P.P. booklet includes:
* The T.A.P.P. Patient Brochure: information on the program requirements, risks of acitretin, and the types of contraceptive methods
* The Contraceptive Counseling Referral Form for female patients who want to receive free contraception counseling reimbursed by the manufacturer
* The Patient Agreement/Informed Consent Form for female patients
* Medication Guide
The T.A.P.P. also includes a voluntary patient survey for women of childbearing potential to assess the effectiveness of the Acitretin Pregnancy Prevention Program T.A.P.P.
T.A.P.P. program materials are available at http://www.tevagenerics.com/acitretin or may be requested by calling 1-855-850-2138.
Information for Patients
See Medication Guide for all patients and Patient Agreement/Informed Consent for Female Patients at end of professional labeling.
Patients should be instructed to read the Medication Guide supplied as required by law when acitretin capsules are dispensed.
Females of Reproductive Potential
Acitretin can cause severe birth defects. Female patients must not be pregnant when therapy with acitretin is initiated, they must not become pregnant while taking acitretin, and for at least 3 years after stopping acitretin, so that the drug can be eliminated to below a blood concentration that would be associated with an increased incidence of birth defects. Because this threshold has not been established for acitretin in humans and because elimination rates vary among patients, the duration of posttherapy contraception to achieve adequate elimination cannot be calculated precisely (see boxed CONTRAINDICATIONS AND WARNINGS ).
Females of reproductive potential should also be advised that they must not ingest beverages or products containing ethanol while taking acitretin and for 2 months after acitretin has been discontinued. This allows for elimination of the acitretin which can be converted to etretinate in the presence of alcohol.
Female patients should be advised that any method of birth control can fail, including tubal ligation, and that microdosed progestin “minipill” preparations are not recommended for use with acitretin (see CLINICAL PHARMACOLOGY, Pharmacokinetic Drug Interactions ). Data from one patient who received a very low-dosed progestin contraceptive (levonorgestrel 0.03 mg) had a significant increase of the progesterone level after 3 menstrual cycles during acitretin treatment.2
Female patients should sign a consent form prior to beginning therapy with acitretin (see boxed CONTRAINDICATIONS AND WARNINGS ).
Nursing Mothers
Studies on lactating rats have shown that etretinate is excreted in the milk. There is one prospective case report where acitretin is reported to be excreted in human milk. Therefore, nursing mothers should not receive acitretin prior to or during nursing because of the potential for serious adverse reactions in nursing infants.
All Patients
Depression and/or other psychiatric symptoms such as aggressive feelings or thoughts of self-harm have been reported. These events, including self-injurious behavior, have been reported in patients taking other systemically administered retinoids, as well as in patients taking acitretin. Since other factors may have contributed to these events, it is not known if they are related to acitretin. Patients should be counseled to stop taking acitretin and notify their prescriber immediately if they experience psychiatric symptoms.
Patients should be advised that a transient worsening of psoriasis is sometimes seen during the initial treatment period. Patients should be advised that they may have to wait 2 to 3 months before they get the full benefit of acitretin, although some patients may achieve significant improvements within the first 8 weeks of treatment as demonstrated in clinical trials.
Decreased night vision has been reported during therapy with acitretin. Patients should be advised of this potential problem and warned to be cautious when driving or operating any vehicle at night. Visual problems should be carefully monitored (see WARNINGS and ADVERSE REACTIONS ). Patients should be advised that they may experience decreased tolerance to contact lenses during the treatment period and sometimes after treatment has stopped.
Patients should not donate blood during and for at least 3 years following therapy because acitretin can cause birth defects and women of childbearing potential must not receive blood from patients being treated with acitretin.
Because of the relationship of acitretin to vitamin A, patients should be advised against taking vitamin A supplements in excess of minimum recommended daily allowances to avoid possible additive toxic effects.
Patients should avoid the use of sun lamps and excessive exposure to sunlight (non-medical UV exposure) because the effects of UV light are enhanced by retinoids.
Patients should be advised that they must not give their acitretin capsules to any other person.
For Prescribers
Acitretin has not been studied in and is not indicated for treatment of acne.
Phototherapy
Significantly lower doses of phototherapy are required when acitretin is used because effects on the stratum corneum induced by acitretin can increase the risk of erythema (burning) (see DOSAGE AND ADMINISTRATION ).
Drug Interactions
Ethanol
Clinical evidence has shown that etretinate can be formed with concurrent ingestion of acitretin and ethanol (see boxed CONTRAINDICATIONS AND WARNINGS and CLINICAL PHARMACOLOGY, Pharmacokinetics ).
Glyburide
In a trial of 7 healthy male volunteers, acitretin treatment potentiated the blood glucose-lowering effect of glyburide (a sulfonylurea similar to chlorpropamide) in 3 of the 7 subjects. Repeating the trial with 6 healthy male volunteers in the absence of glyburide did not detect an effect of acitretin on glucose tolerance. Careful supervision of diabetic patients under treatment with acitretin is recommended (see CLINICAL PHARMACOLOGY, Pharmacokinetics and DOSAGE AND ADMINISTRATION ).
Hormonal Contraceptives
It has not been established if there is a pharmacokinetic interaction between acitretin and combined oral contraceptives. However, it has been established that acitretin interferes with the contraceptive effect of microdosed progestin “minipill” preparations. Microdosed “minipill” progestin preparations are not recommended for use with acitretin (see CLINICAL PHARMACOLOGY, Pharmacokinetic Drug Interactions ). It is not known whether other progestational contraceptives, such as implants and injectables, are adequate methods of contraception during acitretin therapy.
Methotrexate
An increased risk of hepatitis has been reported to result from combined use of methotrexate and etretinate. Consequently, the combination of methotrexate with acitretin is also contraindicated (see CONTRAINDICATIONS ).
Phenytoin
If acitretin is given concurrently with phenytoin, the protein binding of phenytoin may be reduced.
Tetracyclines
Since both acitretin and tetracyclines can cause increased intracranial pressure, their combined use is contraindicated (see CONTRAINDICATIONS and WARNINGS , Pseudotumor Cerebri ).
Vitamin A and Oral Retinoids
Concomitant administration of vitamin A and/or other oral retinoids with acitretin must be avoided because of the risk of hypervitaminosis A.
Other
There appears to be no pharmacokinetic interaction between acitretin and cimetidine, digoxin, or glyburide. Investigations into the effect of acitretin on the protein binding of anticoagulants of the coumarin type (warfarin) revealed no interaction.
Laboratory Tests
If significant abnormal laboratory results are obtained, either dosage reduction with careful monitoring or treatment discontinuation is recommended, depending on clinical judgment.
Blood Sugar
Some patients receiving retinoids have experienced problems with blood sugar control. In addition, new cases of diabetes have been diagnosed during retinoid therapy, including diabetic ketoacidosis. In diabetics, blood-sugar levels should be monitored very carefully.
Lipids
In clinical trials, the incidence of hypertriglyceridemia was 66%, hypercholesterolemia was 33%, and that of decreased HDL was 40%. Pretreatment and follow-up measurements should be obtained under fasting conditions. It is recommended that these tests be performed weekly or every other week until the lipid response to acitretin has stabilized (see WARNINGS ).
Liver Function Tests
Elevations of AST (SGOT), ALT (SGPT), or LDH were experienced by approximately 1 in 3 patients treated with acitretin. It is recommended that these tests be performed prior to initiation of therapy with acitretin, at 1 to 2 week intervals until stable, and thereafter at intervals as clinically indicated (see CONTRAINDICATIONS and boxed WARNINGS ).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
A carcinogenesis study of acitretin in Wistar rats, at doses up to 2 mg/kg/day administered 7 days/week for 104 weeks, has been completed. There were no neoplastic lesions observed that were considered to have been related to treatment with acitretin. An 80 week carcinogenesis study in mice has been completed with etretinate, the ethyl ester of acitretin. Blood level data obtained during this study demonstrated that etretinate was metabolized to acitretin and that blood levels of acitretin exceeded those of etretinate at all times studied. In the etretinate study, an increased incidence of blood vessel tumors (hemangiomas and hemangiosarcomas at several different sites) was noted in male, but not female, mice at doses approximately one-half the maximum recommended human therapeutic dose based on a mg/m2 comparison.
Mutagenesis
Acitretin was evaluated for mutagenic potential in the Ames test, in the Chinese hamster (V79/HGPRT) assay, in unscheduled DNA synthesis assays using rat hepatocytes and human fibroblasts, and in an in vivo mouse micronucleus assay. No evidence of mutagenicity of acitretin was demonstrated in any of these assays.
Impairment of Fertility
In a fertility study in rats, the fertility of treated animals was not impaired at the highest dosage of acitretin tested, 3 mg/kg/day (approximately one-half the maximum recommended therapeutic dose based on a mg/m2 comparison). Chronic toxicity studies in dogs revealed testicular changes (reversible mild to moderate spermatogenic arrest and appearance of multinucleated giant cells) in the highest dosage group (50 then 30 mg/kg/day).
No decreases in sperm count or concentration and no changes in sperm motility or morphology were noted in 31 men (17 psoriatic subjects, 8 subjects with disorders of keratinization, and 6 healthy volunteers) given 30 to 50 mg/day of acitretin for at least 12 weeks. In these trials, no deleterious effects were seen on either testosterone production, LH, or FSH in any of the 31 men.4-6 No deleterious effects were seen on the hypothalamic-pituitary axis in any of the 18 men where it was measured.4,5
Pregnancy
Teratogenic Effects
Pregnancy Category X
(See boxed CONTRAINDICATIONS AND WARNINGS ).
In a study in which acitretin was administered to male rats only at a dosage of 5 mg/kg/day for 10 weeks (approximate duration of one spermatogenic cycle) prior to and during mating with untreated female rats, no teratogenic effects were observed in the progeny (see boxed CONTRAINDICATIONS AND WARNINGS for information about male use of acitretin).
Nonteratogenic Effects
In rats dosed at 3 mg/kg/day (approximately one-half the maximum recommended therapeutic dose based on a mg/m2 comparison), slightly decreased pup survival and delayed incisor eruption were noted. At the next lowest dose tested, 1 mg/kg/day, no treatment-related adverse effects were observed.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. No clinical trials have been conducted in pediatric subjects. Ossification of interosseous ligaments and tendons of the extremities, skeletal hyperostoses, decreases in bone mineral density, and premature epiphyseal closure have been reported in children taking other systemic retinoids, including etretinate, a metabolite of acitretin. A causal relationship between these effects and acitretin has not been established. While it is not known that these occurrences are more severe or more frequent in children, there is special concern in pediatric patients because of the implications for growth potential (see WARNINGS, Hyperostosis ).
Geriatric Use
Clinical trials of acitretin did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. A 2 fold increase in acitretin plasma concentrations was seen in healthy elderly subjects compared with young subjects, although the elimination half-life did not change (see CLINICAL PHARMACOLOGY, Special Populations ).
ACITRETIN ADVERSE REACTIONS
Hypervitaminosis A produces a wide spectrum of signs and symptoms primarily of the mucocutaneous, musculoskeletal, hepatic, neuropsychiatric, and central nervous systems. Many of the clinical adverse reactions reported to date with administration of acitretin resemble those of the hypervitaminosis A syndrome.
Adverse Events/Postmarketing Reports
In addition to the events listed in the tables for the clinical trials, the following adverse events have been identified during postapproval use of acitretin. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular
Acute myocardial infarction, thromboembolism (see WARNINGS ), stroke.
Nervous System
Myopathy with peripheral neuropathy has been reported during therapy with acitretin. Both conditions improved with discontinuation of the drug.
Psychiatric
Aggressive feelings and/or suicidal thoughts have been reported. These events, including self-injurious behavior, have been reported in patients taking other systemically administered retinoids, as well as in patients taking acitretin. Since other factors may have contributed to these events, it is not known if they are related to acitretin (see PRECAUTIONS ).
Reproductive
Vulvo-vaginitis due to Candida albicans.
Skin and Appendages
Thinning of the skin, skin fragility, and scaling may occur all over the body, particularly on the palms and soles; nail fragility is frequently observed.
Clinical Trials
During clinical trials with acitretin, 513/525 (98%) of subjects reported a total of 3,545 adverse events. One-hundred sixteen subjects (22%) left trials prematurely, primarily because of adverse experiences involving the mucous membranes and skin. Three subjects died. Two of the deaths were not drug-related (pancreatic adenocarcinoma and lung cancer); the other subject died of an acute myocardial infarction, considered remotely related to drug therapy. In clinical trials, acitretin was associated with elevations in liver function test results or triglyceride levels and hepatitis.
The tables below list by body system and frequency the adverse events reported during clinical trials of 525 subjects with psoriasis.
| BODY SYSTEM | > 75% | 50% to 75% | 25% to 50% | 10% to 25% |
| CNS | Rigors | |||
| Eye Disorders | Xerophthalmia | |||
| Mucous Membranes | Cheilitis | Rhinitis | Dry mouth Epistaxis | |
| Musculoskeletal |
Arthralgia Spinal hyperostosis (progression of existing lesions) |
|||
| Skin and Appendages |
Alopecia Skin peeling |
Dry skin Nail disorder Pruritus |
Erythematous rash Hyperesthesia Paresthesia Paronychia Skin atrophy Sticky skin |
| BODY SYSTEM | 1% to 10% | < 1% | ||
| Body as a Whole |
Anorexia Edema Fatigue Hot flashes Increased appetite |
Alcohol intolerance Dizziness Fever Influenza-like symptoms |
Malaise Moniliasis Muscle weakness Weight increase |
|
| Cardiovascular | Flushing |
Chest pain Cyanosis Increased bleeding time |
Intermittent claudication Peripheral ischemia |
|
| CNS (also see Psychiatric) |
Headache Pain |
Abnormal gait Migraine Neuritis |
Pseudotumor cerebri (intracranial hypertension) | |
| Eye Disorders |
Abnormal/ blurred vision Blepharitis Conjunctivitis/ irritation Corneal epithelial abnormality |
Decreased night vision/night blindness Eye abnormality Eye pain Photophobia |
Abnormal lacrimation Chalazion Conjunctival hemorrhage Corneal ulceration Diplopia Ectropion |
Itchy eyes and lids Papilledema Recurrent sties Subepithelial corneal lesions |
| Gastrointestinal |
Abdominal pain Diarrhea Nausea Tongue disorder |
Constipation Dyspepsia Esophagitis Gastritis Gastroenteritis |
Glossitis Hemorrhoids Melena Tenesmus Tongue ulceration |
|
| Liver and Biliary |
Hepatic function abnormal Hepatitis Jaundice |
|||
| Mucous Membranes |
Gingival bleeding Gingivitis Increased saliva |
Stomatitis Thirst Ulcerative stomatitis |
Altered saliva Anal disorder Gum hyperplasia |
Hemorrhage Pharyngitis |
| Musculoskeletal |
Arthritis Arthrosis Back pain Hypertonia Myalgia |
Osteodynia Peripheral joint hyperostosis (progression of existing lesions) |
Bone disorder Olecranon bursitis Spinal hyperostosis (new lesions) Tendonitis |
|
| Psychiatric |
Depression Insomnia Somnolence |
Anxiety Dysphonia Libido decreased Nervousness |
||
| Reproductive |
Atrophic vaginitis Leukorrhea |
|||
| Respiratory |
Sinusitis |
Coughing Increased sputum Laryngitis |
||
| Skin and Appendages |
Abnormal skin odor Abnormal hair texture Bullous eruption Cold/clammy skin Dermatitis Increased sweating Infection |
Psoriasiform rash Purpura Pyogenic granuloma Rash Seborrhea Skin fissures Skin ulceration Sunburn |
Acne Breast pain Cyst Eczema Fungal infection Furunculosis Hair discoloration Herpes simplex Hyperkeratosis Hypertrichosis Hypoesthesia Impaired healing Otitis media |
Otitis externa Photosensitivity reaction Psoriasis aggravated Scleroderma Skin nodule Skin hypertrophy Skin disorder Skin irritation Sweat gland disorder Urticaria Verrucae |
| Special Senses/ Other |
Earache Taste perversion Tinnitus |
Ceruminosis Deafness Taste loss |
||
| Urinary |
Abnormal urine Dysuria Penis disorder |
|||
Laboratory
Therapy with acitretin induces changes in liver function tests in a significant number of patients. Elevations of AST (SGOT), ALT (SGPT) or LDH were experienced by approximately 1 in 3 subjects treated with acitretin. In most subjects, elevations were slight to moderate and returned to normal either during continuation of therapy or after cessation of treatment. In subjects receiving acitretin during clinical trials, 66% and 33% experienced elevation in triglycerides and cholesterol, respectively. Decreased high density lipoproteins (HDL) occurred in 40% (see WARNINGS ). Transient, usually reversible elevations of alkaline phosphatase have been observed.
Table 5 lists the laboratory abnormalities reported during clinical trials.
| BODY SYSTEM | 50% to 75% | 25% to 50% | 10% to 25% | 1% to 10% |
| Electrolytes |
Increased: –Phosphorus –Potassium –Sodium Increased and decreased: –Magnesium |
Decreased: –Phosphorus –Potassium –Sodium Increased and decreased: –Calcium –Chloride |
||
| Hematologic |
Increased: –Reticulocytes |
Decreased: –Hematocrit –Hemoglobin –WBC Increased: –Haptoglobin –Neutrophils –WBC |
Increased: –Bands –Basophils –Eosinophils –Hematocrit –Hemoglobin –Lymphocytes –Monocytes Decreased: –Haptoglobin –Lymphocytes –Neutrophils –Reticulocytes Increased or decreased: –Platelets –RBC |
|
| Hepatic |
Increased: –Cholesterol –LDH –SGOT –SGPT Decreased: –HDL cholesterol |
Increased: –Alkaline Phosphatase –Direct bilirubin –GGTP |
Increased: –Globulin –Total bilirubin –Total protein Increased and decreased: –Serum albumin |
|
| Miscellaneous |
Increased: –Triglycerides |
Increased: –CPK –Fasting blood sugar |
Decreased: –Fasting blood sugar –High occult blood |
Increased and decreased: –Iron |
| Renal |
Increased: –Uric acid |
Increased: –BUN –Creatinine |
||
| Urinary | WBC in urine |
Acetonuria Hematuria RBC in urine |
Glycosuria Proteinuria |
OVERDOSAGE
In the event of acute overdosage, acitretin must be withdrawn at once. Symptoms of overdose are identical to acute hypervitaminosis A (e.g., headache and vertigo). The acute oral toxicity (LD50) of acitretin in both mice and rats was greater than 4,000 mg/kg.
In one reported case of overdose, a 32-year-old male with Darier’s disease took 21 x 25 mg capsules (525 mg single dose). He vomited several hours later but experienced no other ill effects.
All female patients of childbearing potential who have taken an overdose of acitretin must:
1) Have a pregnancy test at the time of overdose; 2) Be counseled as per the boxed CONTRAINDICATIONS AND WARNINGS and PRECAUTIONS sections regarding birth defects and contraceptive use for at least 3 years’ duration after the overdose.
ACITRETIN DOSAGE AND ADMINISTRATION
There is intersubject variation in the pharmacokinetics, clinical efficacy, and incidence of side effects with acitretin capsules. A number of the more common side effects are dose-related. Individualization of dosage is required to achieve sufficient therapeutic response while minimizing side effects. Therapy with acitretin capsules should be initiated at 25 to 50 mg per day, given as a single dose with the main meal. Maintenance doses of 25 to 50 mg per day may be given dependent upon an individual patient’s response to initial treatment. Relapses may be treated as outlined for initial therapy.
When acitretin capsules are used with phototherapy, the prescriber should decrease the phototherapy dose, dependent on the patient’s individual response (see PRECAUTIONS ).
Females who have taken Tegison (etretinate) must continue to follow the contraceptive recommendations for Tegison. Tegison is no longer marketed in the U.S.; for information, call 1-888-838-2872.
Information for Pharmacists
An Acitretin Capsules USP Medication Guide must be given to the patient each time acitretin is dispensed, as required by law.
HOW SUPPLIED
Acitretin Capsules USP are available as follows:
10 mg: Two-piece hard gelatin capsule with light green opaque cap and white opaque body filled with yellow powder, imprinted in black ink with TEVA on the cap and 1135 on the body, available in bottles of 30 capsules.
17.5 mg: Two-piece hard gelatin capsule with yellow opaque cap and yellow opaque body filled with yellow powder, imprinted in black ink with "TEVA" on the cap and "1138" on the body, available in bottles of 30 capsules.
25 mg: Two-piece hard gelatin capsule with light green opaque cap and yellow opaque body filled with yellow powder, imprinted in black ink with TEVA on the cap and 1136 on the body, available in bottles of 30 capsules.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP with a child-resistant closure (as required).
PROTECT FROM LIGHT.
AVOID EXPOSURE TO HIGH TEMPERATURES AND HUMIDITY AFTER THE BOTTLE IS OPENED.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
REFERENCES
1. Berbis Ph, et al.: Arch Dermatol Res (1988) 280:388-389. 2. Maier H, Honigsmann H: Concentration of etretinate in plasma and subcutaneous fat after long-term acitretin. Lancet 348:1107, 1996. 3. Geiger JM, Walker M: Is there a reproductive safety risk in male patients treated with acitretin (Neotigason®/Soriatane®)? Dermatology 205:105-107, 2002. 4. Sigg C, et al.: Andrological investigations in patients treated with etretin. Dermatologica 175:48-49, 1987. 5. Parsch EM, et al.: Andrological investigation in men treated with acitretin (Ro 10-1670). Andrologia 22:479-482, 1990. 6. Kadar L, et al.: Spermatological investigations in psoriatic patients treated with acitretin. In: Pharmacology of Retinoids in the Skin; Reichert U. et al., ed, KARGER, Basel, vol. 3, pp 253-254, 1988.
All brand names listed are the registered trademarks of their respective owners and are not trademarks of Teva Pharmaceuticals USA.
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Iss. 2/2014
PATIENT AGREEMENT/INFORMED CONSENT FOR FEMALE PATIENTS
To be completed by the patient* and signed by her prescriber

*Must also be initialed by the parent or guardian of a minor patient (under age 18)
Read each item below and initial in the space provided to show that you understand each item. Do not sign this consent and do not take acitretin capsules if there is anything that you do not understand.
_____________________________________________________________
(Patient’s name)
- I understand that there is a very high risk that my unborn baby could have severe birth defects if I am pregnant or become pregnant while taking acitretin capsules in any amount even for short periods of time. Birth defects have also happened in babies of women who became pregnant after stopping treatment with acitretin capsules.
INITIAL: ___________ - I understand that I must not become pregnant while taking acitretin capsules and for at least 3 years after the end of my treatment with acitretin capsules.
INITIAL: ___________ - I know that I must avoid all alcohol, including drinks, food, medicines, and over-the-counter products that contain alcohol. I understand that the risk of birth defects may last longer than 3 years if I swallow any form of alcohol during therapy with acitretin capsules, and for 2 months after I stop taking acitretin capsules.
INITIAL: ___________ - I understand that I must not have sexual intercourse, or I must use 2 separate, effective forms of birth control at the same time. The only exceptions are if I have had surgery to remove the womb (a hysterectomy) or my prescriber has told me I have gone completely through menopause.
INITIAL: ___________ - I understand that I have to use 2 effective forms of birth control (contraception) at the same time for at least one month before starting acitretin capsules, for the entire time of therapy with acitretin capsules, and for at least 3 years after stopping acitretin capsules.
INITIAL: ___________ - I understand that any form of birth control can fail. Therefore, I must use 2 different methods at the same time, every time I have sexual intercourse.
INITIAL: ___________ - I understand that the following are considered effective forms of birth control: Primary: Tubal ligation (having my tubes tied), partner’s vasectomy, birth control pills, injectable/implantable/insertable/topical (patch) hormonal birth control products, and IUDs (intrauterine devices). Secondary: Latex condoms (with or without spermicide, which is a special cream or jelly that kills sperm), diaphragms and cervical caps (which must be used with a spermicide). I understand that at least 1 of my 2 methods of birth control must be a primary method.
INITIAL: ___________ - I will talk with my prescriber about any medicines or dietary supplements I plan to take while taking acitretin capsules because certain birth control methods may not work if I am taking certain medicines or herbal products (for example, St. John’s wort).
INITIAL: ___________ - Unless I have had a hysterectomy or my prescriber says I have gone completely through menopause, I understand that I must have 2 negative pregnancy test results before I can get a prescription to start acitretin capsules. I will then have pregnancy tests on a monthly basis during therapy with acitretin capsules as instructed by my prescriber. In addition, for at least 3 years after I stop taking acitretin capsules, I will have a pregnancy test every 3 months.
INITIAL: ___________ - I understand that I should not start taking acitretin capsules until I am sure that I am not pregnant and have negative results from 2 pregnancy tests.
INITIAL: ___________ - I have received information on emergency contraception (birth control).
INITIAL: ___________ - I understand that my prescriber can give me a referral for a free contraceptive (birth control) counseling session and pregnancy testing.
INITIAL: ___________ - I understand that on a monthly basis during therapy with acitretin capsules and every 3 months for at least 3 years after stopping acitretin capsules that I should receive counseling from my prescriber about contraception (birth control) and behaviors associated with an increased risk of pregnancy.
INITIAL: ___________ - I understand that I must stop taking acitretin capsules right away and call my prescriber if I get pregnant, miss my menstrual period, stop using birth control, or have sexual intercourse without using my 2 birth control methods during and at least 3 years after stopping acitretin capsules.
INITIAL: ___________ - If I do become pregnant while on acitretin capsules or at any time within 3 years of stopping acitretin capsules, I understand that I should report my pregnancy to TEVA USA, PHARMACOVIGILANCE at 1-866-832-8537 or to the Food and Drug Administration (FDA) MedWatch program at 1-800-FDA-1088. The information I share will be kept confidential (private) and will help the company and the FDA evaluate the pregnancy prevention program to prevent birth defects.
INITIAL: ___________
I have received a copy of the T.A.P.P. brochure. My prescriber has answered all my questions about acitretin capsules. I understand that it is my responsibility to follow my doctor’s instructions, and not to get pregnant during treatment with acitretin capsules or for at least 3 years after I stop taking acitretin capsules.
I now authorize my prescriber, ___________________________________________________, to begin my treatment with acitretin capsules.
Patient signature: ________________________________________
Date: ___________________
Parent/guardian signature (if under age 18): ____________________
Date: ___________________
Please print: Patient name and address: _______________________________________________________________
_______________________________________________________________
Telephone: ______________________________________________________
I have fully explained to the patient, _______________________________________________, the nature and purpose of the treatment described above and the risks to females of childbearing potential. I have asked the patient if she has any questions regarding her treatment with acitretin capsules and have answered those questions to the best of my ability.
Prescriber signature: _______________________________________
Date: __________________
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Iss. 2/2014
MEDICATION GUIDE
ACITRETIN
(A si TRE tin)
CAPSULES
USP
Read this Medication Guide carefully before you start taking acitretin capsules and read it each time you get more acitretin capsules. There may be new information.
The first information in this Guide is about birth defects and how to avoid pregnancy. After this section there is important safety information about possible effects for any patient taking acitretin capsules. ALL patients should read this entire Medication Guide carefully.
This information does not take the place of talking with your prescriber about your medical condition or treatment.
What is the most important information I should know about acitretin capsules?
Acitretin capsules can cause serious side effects, including:
1. SEVERE BIRTH DEFECTS. IF YOU ARE A FEMALE WHO CAN GET PREGNANT, YOU SHOULD USE ACITRETIN CAPSULES ONLY IF YOU ARE NOT PREGNANT NOW, CAN AVOID BECOMING PREGNANT FOR AT LEAST 3 YEARS, AND OTHER MEDICINES DO NOT WORK FOR YOUR SEVERE PSORIASIS OR YOU CANNOT USE OTHER PSORIASIS MEDICINES. INFORMATION ABOUT EFFECTS ON UNBORN BABIES AND ABOUT HOW TO AVOID PREGNANCY IS FOUND IN THE NEXT SECTION: “WHAT ARE THE IMPORTANT WARNINGS AND INSTRUCTIONS FOR FEMALES TAKING ACITRETIN CAPSULES?”

2. Liver problems, including abnormal liver function tests and inflammation of your liver (hepatitis). Your prescriber should do blood tests to check how your liver is working before you start taking and during treatment with acitretin capsules. Stop taking acitretin capsules and call your prescriber right away if you have any of the following signs or symptoms of a serious liver problem:
- yellowing of your skin or the whites of your eyes
- nausea and vomiting
- loss of appetite
- dark urine
What are the important warnings and instructions for females taking acitretin capsules?
- Before you receive your first prescription for acitretin capsules, you should have discussed and signed a Patient Information/Consent form with your prescriber. This is to help make sure you understand the risk of birth defects and how to avoid getting pregnant. If you did not talk to your prescriber about this and sign the form, contact your prescriber.
- You must not take acitretin capsules if you are pregnant or might become pregnant during treatment or at any time for at least 3 years after you stop treatment because acitretin capsules can cause severe birth defects.
- During treatment with acitretin capsules and for 2 months after you stop treatment with acitretin capsules, you must avoid drinks, foods, and all medicines that contain alcohol. This includes over-the-counter products that contain alcohol. Avoiding alcohol is very important, because alcohol changes acitretin capsules into a drug that may take longer than 3 years to leave your body. The chance of birth defects may last longer than 3 years if you swallow any form of alcohol during acitretin capsule therapy and for 2 months after you stop taking acitretin capsules.
-
You and your prescriber must be sure you are not pregnant before you start therapy with acitretin capsules. You must have negative results from 2 pregnancy tests before you start treatment with acitretin capsules. A negative result shows you are not pregnant. Because it takes a few days after pregnancy begins for a test to show that you are pregnant, the first negative test may not ensure you are not pregnant. Do not start acitretin capsules until you have negative results from 2 pregnancy tests.
- The first pregnancy test will be done at the time you and your prescriber decide if acitretin capsules might be right for you.
- The second pregnancy test will usually be done during the first 5 days of your menstrual period, right before you plan to start acitretin capsules. Your prescriber may suggest another time.
- After you start taking acitretin capsules, you must have a pregnancy test repeated each month that you are taking acitretin capsules. This is to be sure that you are not pregnant during treatment because acitretin capsules can cause birth defects.
- For at least 3 years after stopping treatment with acitretin capsules, you must have a pregnancy test repeated every three months to make sure that you are not pregnant.
-
Discuss effective birth control (contraception) with your prescriber. You must use 2 effective forms of birth control (contraception) at the same time during all of the following:
- for at least 1 month before beginning treatment with acitretin capsules
- during treatment with acitretin capsules
- for at least 3 years after stopping treatment with acitretin capsules
-
If you are sexually active, you must use 2 effective forms of birth control (contraception) at the same time even if you think you cannot become pregnant, unless 1 of the following is true for you:
- You had your womb (uterus) removed during an operation (a hysterectomy).
- Your prescriber said you have gone completely through menopause (the “change of life”).
- You can get a free birth control counseling session and pregnancy testing from a prescriber or family planning expert. Your prescriber can give you a Acitretin Capsules Patient Referral Form for this free session.
- You must use 2 effective forms of birth control (contraception) at the same time while you are on treatment with acitretin capsules. You must use birth control for at least 1 month before you start taking acitretin capsules, during treatment, and at least 3 years after you stop treatment with acitretin capsules.
The following are considered effective forms of birth control:
Primary Forms:
- having your tubes tied (tubal ligation)
- partner’s vasectomy
- IUD (intrauterine device)
- birth control pills that contain both estrogen and progestin (combination oral contraceptives)
- hormonal birth control products that are injected, implanted, or inserted in your body
- birth control patch
Secondary Forms (use with a Primary Form):
- diaphragms with spermicide
- latex condoms (with or without spermicide)
- cervical caps with spermicide
At least 1 of your 2 methods of birth control must be a primary form.
- If you have sex at any time without using 2 effective forms of birth control (contraception) at the same time, or if you get pregnant or miss your period, stop using acitretin capsules and call your prescriber right away.
-
Consider “Emergency Contraception” (EC) if you have sex with a male without correctly using 2 effective forms of birth control (contraception) at the same time. EC is also called “emergency birth control” or the “morning after” pill. Contact your prescriber as soon as possible if you have sex without using 2 effective forms of birth control (contraception) at the same time, because EC works best if it is used within 1 or 2 days after sex. EC is not a replacement for your usual 2 effective forms of birth control (contraception) because it is not as effective as regular birth control methods.
You can get EC from private doctors or nurse practitioners, women’s health centers, or hospital emergency rooms. You can get the name and phone number of EC providers nearest you by calling the free Emergency Contraception Hotline at 1-888-668-2528 (1-888-NOT-2-LATE). - Stop taking acitretin capsules right away and contact your prescriber if you get pregnant while taking acitretin capsules or at any time for at least 3 years after treatment has stopped. You need to discuss the possible effects on the unborn baby with your prescriber.
- If you do become pregnant while taking acitretin capsules or at any time for at least 3 years after stopping acitretin capsules, you should report your pregnancy to TEVA USA, PHARMACOVIGILANCE at 1-866-832-8537 or directly to the Food and Drug Administration (FDA) MedWatch program at 1-800-FDA-1088. Your name will be kept in private (confidential). The information you share will help the FDA and the manufacturer evaluate the Pregnancy Prevention Program for acitretin capsules.
- Do not take acitretin capsules if you are breastfeeding. Acitretin can pass into your milk and may harm your baby. You will need to choose either to breastfeed or take acitretin capsules, but not both.
What should males know before taking acitretin capsules?
Small amounts of acitretin are found in the semen of males taking acitretin capsules. Based upon available information, it appears that these small amounts of acitretin in semen pose little, if any, risk to an unborn child while a male patient is taking the drug or after it is discontinued. Discuss any concerns you have about this with your prescriber.
All patients should read the rest of this Medication Guide.
What are acitretin capsules?
Acitretin capsules are a medicine used to treat severe forms of psoriasis in adults. Psoriasis is a skin disease that causes cells in the outer layer of the skin to grow faster than normal and pile up on the skin’s surface. In the most common type of psoriasis, the skin becomes inflamed and produces red, thickened areas, often with silvery scales.
Because acitretin capsules can have serious side effects, you should talk with your prescriber about whether possible benefits of acitretin capsules outweigh their possible risks.
Acitretin capsules may not work right away. You may have to wait 2 to 3 months before you get the full benefit of acitretin capsules. Psoriasis gets worse for some patients when they first start treatment with acitretin capsules.
Acitretin capsules have not been studied in children.
Who should not take acitretin capsules?
- Do NOT take acitretin capsules if you can get pregnant. Do not take acitretin capsules if you are pregnant or might get pregnant during acitretin capsule treatment or at any time for at least 3 years after you stop acitretin capsule treatment (see “What are the important warnings and instructions for females taking acitretin capsules?”).
- Do NOT take acitretin capsules if you are breastfeeding. Acitretin can pass into your milk and may harm your baby. You will need to choose either to breastfeed or take acitretin capsules, but not both.
- Do NOT take acitretin capsules if you have severe liver or kidney disease.
- Do NOT take acitretin capsules if you have repeated high blood lipids (fat in the blood).
-
Do NOT take acitretin capsules if you take these medicines:
- methotrexate
- tetracyclines
The use of these medicines with acitretin capsules may cause serious side effects.
- Do NOT take acitretin capsules if you are allergic to acitretin, the active ingredient in acitretin capsules, to any of the other ingredients (see the end of this Medication Guide for a list of all the ingredients in acitretin capsules), or to any similar medicines (ask your prescriber or pharmacist whether any medicines you are allergic to are related to acitretin capsules).
Tell your prescriber if you have or ever had:
- diabetes or high blood sugar
- liver problems
- kidney problems
- high cholesterol or high triglycerides (fat in the blood)
- heart disease
- depression
- alcoholism
- an allergic reaction to a medication
Your prescriber needs this information to decide if acitretin capsules are right for you and to know what dose is best for you.
Tell your prescriber about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines can cause serious side effects if taken while you also take acitretin capsules. Some medicines may affect how acitretin capsules work, or acitretin capsules may affect how your other medicines work. Be especially sure to tell your prescriber if you are taking the following medicines:
- methotrexate
- tetracyclines
- glyburide
- phenytoin
- vitamin A supplements
- progestin-only oral contraceptives (“minipills”)
- Tegison® or Tigason (etretinate). Tell your prescriber if you have ever taken this medicine in the past.
- St. John’s wort herbal supplement
Tell your prescriber if you are getting phototherapy treatment. Your doses of phototherapy may need to be changed to prevent a burn.
How should I take acitretin capsules?
- Take acitretin capsules with food.
- Be sure to take your medicine as prescribed by your prescriber. The dose of acitretin capsules varies from patient to patient. The number of capsules you must take is chosen specially for you by your prescriber. This dose may change during treatment.
- If you miss a dose, do not double the next dose. Skip the missed dose and resume your normal schedule.
- If you take too many acitretin capsules (overdose), call your local poison control center or emergency room.
You should have blood tests for liver function, cholesterol and triglycerides before starting treatment and during treatment to check your body’s response to acitretin capsules. Your prescriber may also do other tests.
Once you stop taking acitretin capsules, your psoriasis may return. Do not treat this new psoriasis with leftover acitretin capsules. It is important to see your prescriber again for treatment recommendations because your situation may have changed.
What should I avoid while taking acitretin capsules?
- Avoid pregnancy. See “What is the most important information I should know about acitretin capsules?”, and “What are the important warnings and instructions for females taking acitretin capsules?”.
- Avoid breastfeeding. See “What are the important warnings and instructions for females taking acitretin capsules?”.
- Avoid alcohol. Females must avoid drinks, foods, medicines, and over-the-counter products that contain alcohol. The risk of birth defects may continue for longer than 3 years if you swallow any form of alcohol during treatment with acitretin capsules and for 2 months after stopping acitretin capsules (see “What are the important warnings and instructions for females taking acitretin capsules?”).
- Avoid giving blood. Do not donate blood while you are taking acitretin capsules and for at least 3 years after stopping treatment with acitretin capsules. Acitretin in your blood can harm an unborn baby if your blood is given to a pregnant woman. Acitretin capsules do not affect your ability to receive a blood transfusion.
- Avoid progestin-only birth control pills (“minipills”). This type of birth control pill may not work while you take acitretin capsules. Ask your prescriber if you are not sure what type of pills you are using.
- Avoid night driving if you develop any sudden vision problems. Stop taking acitretin capsules and call your prescriber if this occurs (see “Serious side effects”).
- Avoid non-medical ultraviolet (UV) light. Acitretin capsules can make your skin more sensitive to UV light. Do not use sunlamps, and avoid sunlight as much as possible. If you are taking light treatment (phototherapy), your prescriber may need to change your light dosages to avoid burns.
- Avoid dietary supplements containing vitamin A. Acitretin is related to vitamin A. Therefore, do not take supplements containing vitamin A, because they may add to the unwanted effects of acitretin capsules. Check with your prescriber or pharmacist if you have any questions about vitamin supplements.
- DO NOT SHARE acitretin capsules with anyone else, even if they have the same symptoms. Your medicine may harm them or their unborn child.
What are the possible side effects of acitretin capsules?
Acitretin capsules can cause serious side effects. See “What is the most important information I should know about acitretin capsules?” and “ What are the important warnings and instructions for females taking acitretin capsules?”
Stop taking acitretin capsules and call your prescriber right away if you get the following signs or symptoms of possible serious side effects:
- Bad headaches, nausea, vomiting, blurred vision. These symptoms can be signs of increased brain pressure that can lead to blindness or even death.
- Decreased vision in the dark (night blindness). Since this can start suddenly, you should be very careful when driving at night. This problem usually goes away when acitretin capsule treatment stops. If you develop any vision problems or eye pain stop taking acitretin capsules and call your prescriber.
- Depression. There have been some reports of patients developing mental problems including a depressed mood, aggressive feelings, or thoughts of ending their own life (suicide). These events, including suicidal behavior, have been reported in patients taking other drugs similar to acitretin capsules as well as patients taking acitretin capsules. Since other things may have contributed to these problems, it is not known if they are related to acitretin capsules.
- Aches or pains in your bones, joints, muscles, or back; trouble moving; loss of feeling in your hands or feet. These can be signs of abnormal changes to your bones or muscles.
- Frequent urination, great thirst or hunger. Acitretin capsules can affect blood sugar control, even if you do not already have diabetes. These are some of the signs of high blood sugar.
- Shortness of breath, dizziness, nausea, chest pain, weakness, trouble speaking, or swelling of a leg. These may be signs of a heart attack, blood clots, or stroke. Acitretin capsules can cause serious changes in blood fats (lipids). It is possible for these changes to cause blood vessel blockages that lead to heart attacks, strokes, or blood clots.
Common side effects
If you develop any of these side effects or any unusual reaction, check with your prescriber to find out if you need to change the amount of acitretin capsules you take. These side effects usually get better if the dose of acitretin capsules is reduced or acitretin capsules are stopped.
- Chapped lips; peeling fingertips, palms, and soles; itching; scaly skin all over; weak nails; sticky or fragile (weak) skin; runny or dry nose, or nosebleeds. Your prescriber or pharmacist can recommend a lotion or cream to help treat drying or chapping.
- Dry mouth
- Joint pain
- Tight muscles
- Hair loss. Most patients have some hair loss, but this condition varies among patients. No one can tell if you will lose hair, how much hair you may lose or if and when it may grow back.
- Dry eyes. Acitretin capsules may dry your eyes. Wearing contact lenses may be uncomfortable during and after treatment with acitretin capsules because of the dry feeling in your eyes. If this happens, remove your contact lenses and call your prescriber. Also read the section about vision under “Serious side effects”.
- Rise in blood fats (lipids). Acitretin capsules can cause your blood fats (lipids) to rise. Most of the time this is not serious. But sometimes the increase can become a serious problem (see information under “Serious side effects”). You should have blood tests as directed by your prescriber.
Psoriasis gets worse for some patients when they first start treatment with acitretin capsules. Some patients have more redness or itching. If this happens, tell your prescriber. These symptoms usually get better as treatment continues, but your prescriber may need to change the amount of your medicine.
These are not all the possible side effects of acitretin capsules. For more information, ask your prescriber or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store acitretin capsules?
- Keep acitretin capsules away from sunlight, high temperature, and humidity.
- Keep acitretin capsules and all medicines out of the reach of children.
What are the ingredients in acitretin capsules?
Active ingredient: acitretin
Inactive ingredients: crospovidone, microcrystalline cellulose, poloxamer, povidone, sodium ascorbate and sodium lauryl sulfate.
The 10 mg, 17.5, and 25 mg gelatin capsule shells contain gelatin and titanium dioxide. The 10 mg and 25 mg capsule shells also contain D&C yellow no. 10, FD&C blue no. 1, and FD&C red no. 40. The 17.5 mg capsule shells also contain red iron oxide and yellow iron oxide. The 25 mg gelatin capsule shells also contain FD&C yellow no. 6. The edible imprinting ink contains black iron oxide, D&C yellow no. 10 aluminum lake, FD&C blue no. 1 aluminum lake, FD&C blue no. 2 aluminum lake, FD&C red no. 40 aluminum lake, propylene glycol and shellac glaze.
General information about the safe and effective use of acitretin capsules
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use acitretin capsules for a condition for which they were not prescribed. Do not give acitretin capsules to other people, even if they have the same symptoms that you have.
This Medication Guide summarizes the most important information about acitretin capsules. If you would like more information, talk with your prescriber. You can ask your pharmacist or prescriber for information about acitretin capsules that is written for health professionals.
For more information about acitretin capsules, call 1-855-850-2138 or go to http://www.tevagenerics.com/acitretin.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
All brand names listed are the registered trademarks of their respective owners and are not trademarks of Teva Pharmaceuticals USA.
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Iss. 2/2014
PRINCIPAL DISPLAY PANEL

Acitretin Capsules USP 10 mg 30s Label Text
NDC 0093-1135-56
ACITRETIN
Capsules USP, 10 mg
PHARMACIST: Dispense the accompanying
Medication Guide to each patient.
CAUSES BIRTH DEFECTS
DO NOT GET PREGNANT
Rx only
30 CAPSULES
TEVA
PRINCIPAL DISPLAY PANEL

Acitretin Capsules USP 17.5 mg 30s Label Text
NDC 0093-1138-56
ACITRETIN
Capsules USP
17.5 mg
CAUSES BIRTH DEFECTS
DO NOT GET PREGNANT
PHARMACIST: Dispense the accompanying
Medication Guide to each patient.
Rx only
30 CAPSULES
TEVA
PRINCIPAL DISPLAY PANEL

Acitretin Capsules USP 25 mg 30s Label Text
NDC 0093-1136-56
ACITRETIN
Capsules USP, 25 mg
PHARMACIST: Dispense the accompanying
Medication Guide to each patient.
CAUSES BIRTH DEFECTS
DO NOT GET PREGNANT
Rx only
30 CAPSULES
TEVA
AcitretinAcitretin CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AcitretinAcitretin CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AcitretinAcitretin CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||