Acetic Acid
Acetic Acid 2% in AqueousAluminum Acetate Otic Solution
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACETIC ACID DESCRIPTION
- CLINICAL PHARMACOLOGY
- ACETIC ACID INDICATIONS AND USAGE
- ACETIC ACID CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ACETIC ACID ADVERSE REACTIONS
- OVERDOSAGE
- ACETIC ACID DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Storage:
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Rx only
FOR USE IN THE EARS ONLY
NOT FOR USE IN THE EYES
ACETIC ACID DESCRIPTION
Acetic Acid 2% in Aqueous Aluminum Acetate Otic Solution contains acetic acid 2% as the active ingredient, in a solution containing aluminum acetate (formed from Aluminum Sulfate, Calcium Carbonate, Glacial Acetic Acid), Boric Acid, Sodium Hydroxide and Purified Water. Sodium Hydroxide and/or Glacial Acetic Acid may be added to adjust the pH (3.5 to 5.0). Acetic Acid 2% in Aqueous Aluminum Acetate Otic Solution is instilled in the external auditory canal. Acetic acid is an astringent and antimicrobial agent.
Chemically, acetic acid is C2H4O2 and has the following structural formula:
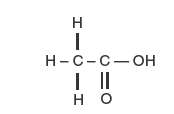
Molecular weight: 60.05
CLINICAL PHARMACOLOGY
Acetic acid is antibacterial and antifungal and is effective against microorganisms (bacteria and fungi) that infect the ears of patients with acute diffuse external otitis. In vitro tests, minimum lethal-time was less than 0.25 minutes when bacteria and fungi isolated from patients with otitis externa were exposed to 2% acetic acid. Quantitative absorption of acetic acid 2% from external auditory canal is not known.
ACETIC ACID INDICATIONS AND USAGE
For the treatment of superficial infections of the external auditory canal caused by organisms susceptible to the action of the antimicrobial.
ACETIC ACID CONTRAINDICATIONS
Hypersensitivity to acetic acid or any of the ingredients in this product. Perforated tympanic membranes is considered a contraindication to the use of any external ear canal medication.
WARNINGS
Avoid use or use with caution in patients with perforated tympanic membrane (See CONTRAINDICATIONS).
PRECAUTIONS
General
Care should be taken to assure that the Acetic Acid 2% in Aqueous Aluminum Acetate Otic Solution gets into the ear canal and stays in contact with the affected area long enough for the drug to act.
Discontinue promptly if sensitization or irritation occurs.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long term studies in animals have been performed to evaluate the carcinogenic potential of Acetic Acid 2% in Aqueous Aluminum Acetate Otic Solution.
ACETIC ACID ADVERSE REACTIONS
Irritation may occur.
OVERDOSAGE
No Toxic effect has been reported with overdosage of Acetic Acid 2% in Aqueous Aluminum Acetate Otic Solution.
ACETIC ACID DOSAGE AND ADMINISTRATION
The patient should lie on the side with the affected ear uppermost. Instill 4 to 6 drops into the external auditory canal and maintain this position for 5 minutes. Repeat the procedure every 2 to 3 hours.
HOW SUPPLIED
Acetic Acid 2% in Aqueous Aluminum Acetate Otic Solution is available in 60 mL plastic squeeze bottle with otic tip.
NDC 24208-615-77
Storage:
Store between 15˚ - 25˚ C (59˚ - 77˚ F).
PROTECT FROM FREEZING
DO NOT USE IF IMPRINTED NECKBAND IS NOT INTACT.
KEEP OUT OF REACH OF CHILDREN.
Revised: November 2012
Bausch & Lomb Incorporated
Tampa, FL 33637
©Bausch & Lomb Incorporated
9066902 Folded
9274302 Flat
Principal Display Panel
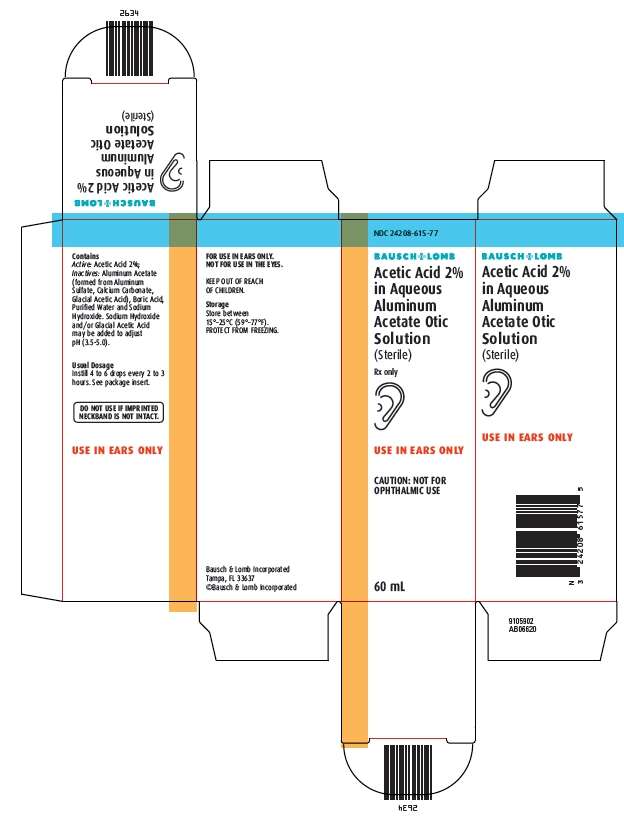
NDC 24208-615-77
Bausch & Lomb
Acetic Acid 2%
in Aqueous
Aluminum
Acetate Otic
Solution
(Sterile)
Rx only
[icon-ear]
USE IN EARS ONLY
CAUTION: NOT FOR OPHTHALMIC USE
60 mL
Acetic AcidAcetic Acid SOLUTION/ DROPS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||