Topiramate
These highlights do not include all the information needed to use topiramate tablets safely and effectively. See full prescribing information for topiramate tablets Topiramate TabletsRx Only Initial U.S. Approval - 1996
FULL PRESCRIBING INFORMATION: CONTENTS*
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- DOSAGE FORMS & STRENGTHS
- TOPIRAMATE CONTRAINDICATIONS
- WARNINGS AND PRECAUTIONS
- TOPIRAMATE ADVERSE REACTIONS
- DRUG INTERACTIONS
- USE IN SPECIFIC POPULATIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- TOPIRAMATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- NONCLINICAL TOXICOLOGY
- CLINICAL STUDIES
- INFORMATION FOR PATIENTS
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
INDICATIONS & USAGE
1. INDICATIONS AND USAGE1.1 Monotherapy Epilepsy
14.1
1.2 Adjunctive Therapy Epilepsy
14.2
DOSAGE & ADMINISTRATION
2.1 Epilepsy14.1
14.1
2.4 Patients with Renal Impairment
2.5 Geriatric Patients (Ages 65 Years and Over)
12.3
2.6 Patients Undergoing Hemodialysis
2.7 Patients with Hepatic Disease
DOSAGE FORMS & STRENGTHS
3. DOSAGE FORMS AND STRENGTHSTOPIRAMATE CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
5. WARNINGS AND PRECAUTIONS5.1 Acute Myopia and Secondary Angle Closure Glaucoma
5.2 Oligohidrosis and Hyperthermia
5.3 Suicidal Behavior and Ideation
5.4 Metabolic Acidosis
8.4
8.4
5.5 Cognitive/Neuropsychiatric Adverse Reactions
6
5.3
5.6 Withdrawal of Antiepileptic Drugs (AEDs)
14
5.7 Sudden Unexplained Death in Epilepsy (SUDEP)
5.8 Hyperammonemia and Encephalopathy (Without and With Concomitant Valproic Acid [VPA] Use) Hyperammonemia/Encephalopathy Without Concomitant Valproic Acid (VPA)
8.4
5.9 Kidney Stones
8.4
5.4
5.10 Paresthesia
5.11 Adjustment of Dose in Renal Failure
2
5.12 Decreased Hepatic Function
5.13 Monitoring: Laboratory Tests
5.4
8.4
5.8
TOPIRAMATE ADVERSE REACTIONS
6. ADVERSE REACTIONS6.1 Monotherapy Epilepsy
Table 2
Table 3
Body System/Body as a Whole - General DisordersCentral & Peripheral Nervous System DisordersGastro-Intestinal System DisordersLiver and Biliary System DisordersMetabolic and Nutritional DisordersPsychiatric DisordersReproductive Disorders, FemaleRed Blood Cell DisordersResistance Mechanism DisordersRespiratory System DisordersSkin and Appendages DisordersSpecial Senses Other, DisordersUrinary System Disorders
Body System/Body as a Whole-General DisordersCentral & Peripheral Nervous System DisordersGastro-Intestinal System DisordersMetabolic and Nutritional DisordersPsychiatric DisordersResistance Mechanism DisordersRespiratory System DisordersSkin and Appendages Disorders
6.2 Adjunctive Therapy Epilepsy
Table 4Table 6
Table 7
6.3 Incidence in Epilepsy Controlled Clinical TrialsAdjunctive TherapyPartial Onset Seizures, Primary Generalized Tonic-Clonic Seizures, and Lennox-Gastaut Syndrome
6.4 Other Adverse Reactions Observed During Double-Blind Epilepsy Adjunctive Therapy Trials
Body System/Body as a Whole-General DisordersCentral & Peripheral Nervous System DisordersGastro-Intestinal System DisordersHearing and Vestibular DisordersMetabolic and Nutritional DisordersMuscle-Skeletal System DisordersPlatelet, Bleeding, & Clotting DisordersPsychiatric DisordersReproductive Disorders, FemaleReproductive Disorders, MaleResistance Mechanism DisordersRespiratory System DisordersSkin and Appendages DisordersSpecial Sense Other, DisordersUrinary System DisordersVision DisordersWhite Cell and RES Disorders
6.5 Incidence in Study 119Add-On TherapyAdults with Partial Onset Seizures
Topiramate Tablets Dosage(mg/day)Body System/Placebo200Adverse Reaction c(N = 92)(N = 171)Body as a Whole-General DisordersCardiovascular Disorders, GeneralCentral & Peripheral Nervous System DisordersGastro-Intestinal System DisordersHearing and Vestibular DisordersMetabolic and Nutritional DisordersRespiratory System DisordersUrinary System DisordersVision Disorders
Body System/Body as a Whole - General DisordersCardiovascular Disorders, GeneralCentral & Peripheral Nervous System DisordersGastro-Intestinal System DisordersMetabolic and Nutritional DisordersPlatelet, Bleeding, & Clotting DisordersPsychiatric DisordersReproductive Disorders, FemaleResistance Mechanism DisordersRespiratory System DisordersSkin and Appendages DisordersUrinary System DisordersVision DisordersWhite Cell and RES Disorders
6.6 Other Adverse Reactions Observed During All Epilepsy Clinical Trials
6.9 Postmarketing and Other Experience
DRUG INTERACTIONS
7. DRUG INTERACTIONS12.5
7.1 Antiepileptic Drugs
12.55.812.5
7.2 CNS Depressants
7.3 Oral Contraceptives
12.5
7.4 Metformin
12.5
7.5 Lithium
12.5
7.6 Other Carbonic Anhydrase Inhibitors
12.5
USE IN SPECIFIC POPULATIONS
8. USE IN SPECIFIC POPULATIONS8.1 Pregnancy
5.4'ability to tolerate labor. Pregnant patients should be monitored for metabolic acidosis and treated as in the nonpregnant state [see Warnings and Precautions (5.4)]. Newborns of mothers treated with topiramate should be monitored for metabolic acidosis because of transfer of topiramate to the fetus and possible occurrence of transient metabolic acidosis following birth.
There are no studies using topiramate in pregnant women. Topiramate should be used during pregnancy only if the potential benefit outweighs the potential risk to the fetus.
Topiramate has demonstrated selective developmental toxicity, including teratogenicity, in multiple animal species at clinically relevant doses. When oral doses of 20, 100 or 500 mg/kg were administered to pregnant mice during the period of organogenesis, the incidence of fetal malformations (primarily craniofacial defects) was increased at all doses. The low dose is approximately 0.2 times the recommended human dose (RHD) 400 mg/day on a mg/m2 basis. Fetal body weights and skeletal ossification were reduced at 500 mg/kg in conjunction with decreased maternal body weight gain.
In rat studies (oral doses of 20, 100, and 500 mg/kg or 0.2, 2.5, 30, and 400 mg/kg), the frequency of limb malformations (ectrodactyly, micromelia, and amelia) was increased among the offspring of dams treated with 400 mg/kg (10 times the RHD on a mg/m2 basis) or greater during the organogenesis period of pregnancy. Embryotoxicity (reduced fetal body weights, increased incidence of structural variations) was observed at doses as low as 20 mg/kg (0.5 times the RHD on a mg/m2 basis). Clinical signs of maternal toxicity were seen at 400 mg/kg and above, and maternal body weight gain was reduced during treatment with 100 mg/kg or greater.
In rabbit studies (20, 60, and 180 mg/kg or 10, 35, and 120 mg/kg orally during organogenesis), embryo/fetal mortality was increased at 35 mg/kg (2 times the RHD on a mg/m2 basis) or greater, and teratogenic effects (primarily rib and vertebral malformations) were observed at 120 mg/kg (6 times the RHD on a mg/m2 basis). Evidence of maternal toxicity (decreased body weight gain, clinical signs, and/or mortality) was seen at 35 mg/kg and above.
When female rats were treated during the latter part of gestation and throughout lactation (0.2, 4, 20, and 100 mg/kg or 2, 20, and 200 mg/kg), offspring exhibited decreased viability and delayed physical development at 200 mg/kg (5 times the RHD on a mg/m2 basis) and reductions in pre-and/or postweaning body weight gain at 2 mg/kg (0.05 times the RHD on a mg/m2 basis) and above. Maternal toxicity (decreased body weight gain, clinical signs) was evident at 100 mg/kg or greater.
In a rat embryo/fetal development study with a postnatal component (0.2, 2.5, 30 or 400 mg/kg during organogenesis; noted above), pups exhibited delayed physical development at 400 mg/kg (10 times the RHD on a mg/m2 basis) and persistent reductions in body weight gain at 30 mg/kg (1 times the RHD on a mg/m2 basis) and higher.
Pregnancy Registry
The North American Drug Pregnancy Registry has been established to collect information and provide scientific knowledge about safety and outcomes associated with pregnant women being treated with antiepileptic drugs. It is desirable that the experience from patients who are exposed to topiramate during pregnancy be reported to this registry. Such information can be reported to the North American Drug Pregnancy Registry by either a healthcare provider or the patient by calling 1-888-233-2334. Information about the North American Drug Pregnancy Registry can be found at http://www.massgeneral.org/aed/ .
8.2 Labor and Delivery
Although the effect of topiramate on labor and delivery in humans has not been established, the development of topiramate-induced metabolic acidosis in the mother and/or in the fetus might affect the fetus'ability to tolerate labor [see Pregnancy (8.1)].
8.3 Nursing Mothers
Limited data on 5 breastfeeding infants exposed to topiramate showed infant plasma topiramate levels equal to 10-20% of the maternal plasma level. The effects of this exposure on infants are unknown. Caution should be exercised when administered to a nursing woman.
8.4 Pediatric Use
Adjunctive Treatment for Partial Onset Epilepsy in Infants and Toddlers (1 to 24 months)
Safety and effectiveness in patients below the age of 2 years have not been established for the adjunctive therapy treatment of partial onset seizures, primary generalized tonic-clonic seizures, or seizures associated with Lennox-Gastaut syndrome. In a single randomized, double-blind placebo-controlled investigational trial, the efficacy, safety, and tolerability of topiramate oral liquid and sprinkle formulations as an adjunct to concurrent antiepileptic drug therapy in infants 1 to 24 months of age with refractory partial onset seizures, was assessed. After 20 days of double-blind treatment, topiramate (at fixed doses of 5, 15, and 25 mg/kg per day) did not demonstrate efficacy compared with placebo in controlling seizures.
In general, the adverse reaction profile in this population was similar to that of older pediatric patients, although results from the above controlled study, and an open-label long-term extension study in these infants/toddlers (1 to 24 months old) suggested some adverse reactions/toxicities (not previously observed in older pediatric patients and adults; i.e, growth/length retardation, certain clinical laboratory abnormalities, and other adverse reactions/toxicities that occurred with a greater frequency and/or greater severity than had been recognized previously from studies in older pediatric patients or adults for various indications.
These very young pediatric patients appeared to experience an increased risk for infections (any topiramate dose 12%, placebo 0%) and of respiratory disorders (any topiramate dose 40%, placebo 16%). The following adverse reactions were observed in at least 3% of patients on topiramate and were 3% to 7% more frequent then in patients on placebo: viral infection, bronchitis, pharyngitis, rhinitis, otitis media, upper respiratory infection, cough, and bronchospasm. A generally similar profile was observed in older children [see Adverse Reactions (6)].
Topiramate resulted in an increased incidence of patients with increased creatinine (any topiramate dose 5%, placebo 0%), BUN (any topiramate dose 3%, placebo 0%), and protein (any topiramate dose 34%, placebo 6%), and an increased incidence of decreased potassium (any topiramate dose 7%, placebo 0%). This increased frequency of abnormal values was not dose-related. Creatinine was the only analyte showing a noteworthy increased incidence (topiramate 25 mg/kg/d 5%, placebo 0%) of a markedly abnormal increase [see Warnings and Precautions (5.13)]. The significance of these finding is uncertain.
Topiramate treatment also produced a dose-related increase in the percentage of patients who had a shift from normal at baseline to high/increased (above the normal reference range) in total eosinophil count at the end of treatment. The incidence of these abnormal shifts was 6 % for placebo, 10% for 5 mg/kg/d, 9% for 15 mg/kg/d, 14% for 25 mg/kg/d, and 11% for any topiramate dose [see Warnings and Precautions (5.13)]. There was a mean dose-related increase in alkaline phosphatase. The significance of these finding is uncertain.
Topiramate produced a dose-related increased incidence of treatment-emergent hyperammonemia. [see Warnings and Precautions (5.8)].
Treatment with topiramate for up to 1 year was associated with reductions in Z SCORES for length, weight, and head circumference. [see Warnings and Precautions (5.4) and Adverse Reactions (6)].
In open-label, uncontrolled experience, increasing impairment of adaptive behavior was documented in behavioral testing over time in this population. There was a suggestion that this effect was dose-related. However, because of the absence of an appropriate control group, it is not known if this decrement in function was treatment related or reflects the patient's underlying disease (e.g., patients who received higher doses may have more severe underlying disease) [see Warnings and Precautions (5.5)].
In this open-label, uncontrolled study, the mortality was 37 deaths/1000 patient years. It is not possible to know whether this mortality rate is related to topiramate treatment, because the background mortality rate for a similar, significantly refractory, young pediatric population (1-24 months) with partial epilepsy is not known.
Monotherapy Treatment in Partial Onset Epilepsy in Patients <10 Years Old
Safety and effectiveness in patients below the age of 10 years have not been established for the monotherapy treatment of epilepsy.
Juvenile Animal Studies
When topiramate (30, 90 or 300 mg/kg/day) was administered orally to rats during the juvenile period of development (postnatal days 12 to 50), bone growth plate thickness was reduced in males at the highest dose, which is approximately 5-8 times the maximum recommended pediatric dose (9 mg/kg/day) on a body surface area (mg/m2) basis.
8.5 Geriatric Use
In clinical trials, 3% of patients were over 60. No age related difference in effectiveness or adverse effects were evident. However, clinical studies of topiramate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. Dosage adjustment may be necessary for elderly with impaired renal function (creatinine clearance rate <70 mL/min/1.73 m2) due to reduced clearance of topiramate [see Clinical Pharmacology (12.3) and Dosage and Administration (2.5)].
8.6 Race and Gender Effects
Evaluation of effectiveness and safety in clinical trials has shown no race or gender related effects.
8.7 Renal Impairment
The clearance of topiramate was reduced by 42% in moderately renally impaired (creatinine clearance 30 to 69 mL/min/1.73 m2) and by 54% in severely renally impaired subjects (creatinine clearance <30 mL/min/1.73 m2) compared to normal renal function subjects (creatinine clearance >70 mL/min/1.73 m2). One-half the usual starting and maintenance dose is recommended in patients with moderate or severe renal impairment [see Dosage and Administration (2.6) and Clinical Pharmacology (12.4)].
8.8 Patients Undergoing Hemodialysis
Topiramate is cleared by hemodialysis at a rate that is 4 to 6 times greater than a normal individual. Accordingly, a prolonged period of dialysis may cause topiramate concentration to fall below that required to maintain an anti-seizure effect. To avoid rapid drops in topiramate plasma concentration during hemodialysis, a supplemental dose of topiramate may be required. The actual adjustment should take into account the duration of dialysis period, the clearance rate of the dialysis system being used, and the effective renal clearance of topiramate in the patient being dialyzed [see Dosage and Administration (2.4) and Clinical Pharmacology (12.4)].
DRUG ABUSE AND DEPENDENCE
9. DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
OVERDOSAGE
10. OVERDOSAGE5.4
TOPIRAMATE DESCRIPTION
11. DESCRIPTION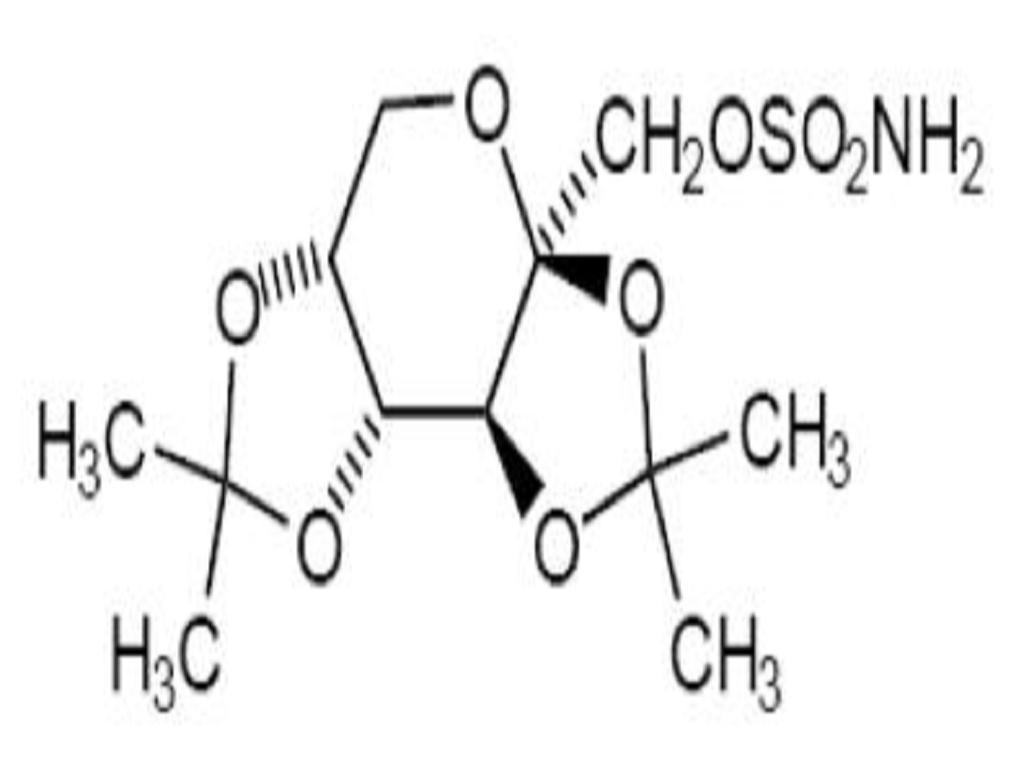
CLINICAL PHARMACOLOGY
12. CLINICAL PHARMACOLOGY12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Special Populations
2.42.55.11
2.6
2.7
2.45.11
12.5 Drug-Drug Interactions
5.87.1
7.2
7.3
7.4
7.5
7.5
NONCLINICAL TOXICOLOGY
13. NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
CLINICAL STUDIES
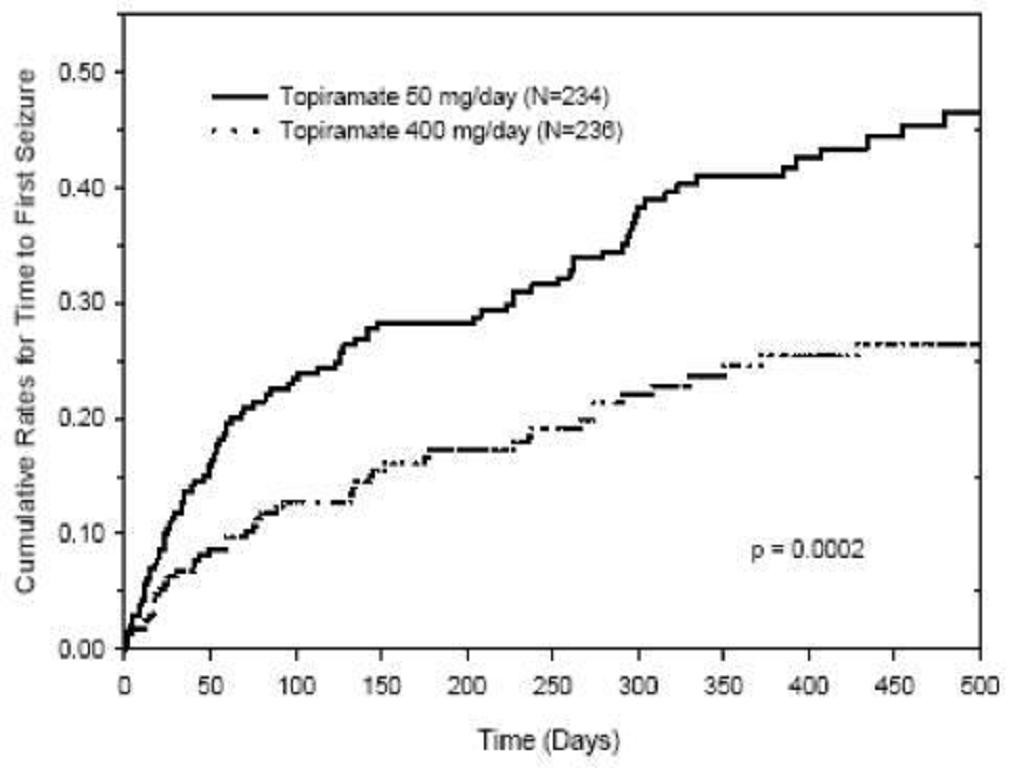
14. CLINICAL STUDIES14.1 Monotherapy Epilepsy Controlled Trial
16. HOW SUPPLIED/STORAGE AND HANDLING
Topiramate tablets
Storage and Handling
INFORMATION FOR PATIENTS
17. PATIENT COUNSELING INFORMATIONFDA approved Medication Guide.
17.1 Eye Disorders
5.1
17.2 Oligohydrosis and Hyperthermia
5.2
17.3 Suicidal Behavior and Ideation
17.4 Metabolic Acidosis
5.4
17.5 Interference with Cognitive and Motor Performance
5.5
17.6 Hyperammonemia and Encephalopathy
5.8
17.7 Kidney Stones
5.9
17.8 Use in Pregnancy
8.18.38.1
SPL MEDGUIDE
-
● any sudden decrease in vision with or without eye pain and redness,
-
● a blockage of fluid in the eye causing increased pressure in the eye (secondary angle closure glaucoma).
-
● These eye problems can lead to permanent loss of vision if not treated. You should call your healthcare provider right away if you have any new eye symptoms.
-
● thoughts about suicide or dying
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
-
● Stopping topiramate tablets suddenly can cause serious problems.
-
● Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
-
● Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
-
● Keep all follow-up visits with your healthcare provider as scheduled.
-
● Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
-
● to treat certain types of seizures (partial onset seizures and primary generalized tonic-clonic seizures) in people 10 years and older,
-
● with other medicines to treat certain types of seizures (partial onset seizures, primary generalized tonic-clonic seizures, and seizures associated with Lennox-Gastaut syndrome) in adults and children 2 years and older.
-
● have or have had depression, mood problems or suicidal thoughts or behavior
-
● have kidney problems, kidney stones or are getting kidney dialysis
-
● have a history of metabolic acidosis (too much acid in the blood)
-
● have liver problems
-
● have osteoporosis, soft bones, or decreased bone density
-
● have lung or breathing problems
-
● have eye problems, especially glaucoma
-
● have diarrhea
-
● have a growth problem
-
● are on a diet high in fat and low in carbohydrates, which is called a ketogenic diet
-
● are having surgery
-
● are pregnant or plan to become pregnant. It is not known if topiramate tablets will harm your unborn baby. If you become pregnant while taking topiramate tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic medicine during pregnancy.
-
● are breastfeeding. It is not known if topiramate tablets passes into breast milk and if it can harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take topiramate tablets.
-
● Valproic acid
-
● any medicines that impair or decrease your thinking, concentration, or muscle coordination.
-
● birth control pills. Topiramate tablets may make your birth control pills less effective. Tell your healthcare provider if your menstrual bleeding changes while you are taking birth control pills and topiramate tablets.
-
● Take topiramate tablets exactly as prescribed.
-
● Your healthcare provider may change your dose. Do not change your dose without talking to your healthcare provider.
-
● Topiramate tablets should be swallowed whole. Do not chew the tablets. They may leave a bitter taste.
-
● Do not store any medicine and food mixture for later use.
-
● Topiramate tablets can be taken before, during, or after a meal. Drink plenty of fluids during the day. This may help prevent kidney stones while taking topiramate tablets.
-
● If you take too much topiramate tablets, call your healthcare provider or poison control center right away or go to the nearest emergency room.
-
● If you miss a single dose of topiramate tablets, take it as soon as you can. However, if you are within 6 hours of taking your next scheduled dose, wait until then to take your usual dose of topiramate tablets, and skip the missed dose. Do not double your dose. If you have missed more than one dose, you should call your healthcare professional for advice.
-
● Do not stop taking topiramate tablets without talking to your healthcare provider. Stopping topiramate tablets suddenly may cause serious problems. If you have epilepsy and you stop taking topiramate tablets suddenly, you may have seizures that do not stop. Your healthcare provider will tell you how to stop taking topiramate tablets slowly.
-
● Your healthcare provider may do blood tests while you take topiramate tablets.
-
● Do not drink alcohol while taking topiramate tablets. Topiramate tablets and alcohol can affect each other causing side effects such as sleepiness and dizziness.
-
● Do not drive a car or operate heavy machinery until you know how topiramate tablet affects you. Topiramate tablets can slow your thinking and motor skills, and/or vision.
-
● tiredness
-
● loss of appetite
-
● irregular heartbeat
-
● impaired consciousness
-
● tingling of the arms and legs (paresthesia)
-
● not feeling hungry
-
● nausea
-
● a change in the way foods taste
-
● diarrhea
-
● weight loss
-
● nervousness
-
● upper respiratory tract infection
-
● Store topiramate tablets at 20- 25(68- 77- 30(59- 86[see USP Controlled Room Temperature]. PROTECT FROM MOISTURE.
-
● Keep topiramate tablets in a tightly closed container.
-
● Keep topiramate tablets and all medicines out of the reach of children.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

TopiramateTopiramate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!