Tamsulosin Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- SPL INDEXING DATA ELEMENTS
- 1.0 INDICATIONS & USAGE
- 2.0 DOSAGE & ADMINISTRATION
- 3.0 DOSAGE FORMS & STRENGTHS
- 4.0 TAMSULOSIN HYDROCHLORIDE CONTRAINDICATIONS
- 5.0 WARNINGS
- 6.0 TAMSULOSIN HYDROCHLORIDE ADVERSE REACTIONS
- 7.0 DRUG INTERACTIONS
- 8.0 USE IN SPECIFIC POPULATIONS
- 9.0 OVERDOSAGE
- 10.0 TAMSULOSIN HYDROCHLORIDE DESCRIPTION
- 12.0 CLINICAL PHARMACOLOGY
- 13.0 CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- 14.0 CLINICAL STUDIES
- 15.0 HOW SUPPLIED
- 17.0 SPL PATIENT PACKAGE INSERT
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
SPL INDEXING DATA ELEMENTS
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use tamsulosin hydrochloride capsules safely and effectively. See full prescribing information for tamsulosin hydrochloride capsules.Tamsulosin Hydrochloride Capsules, 0.4 mg
Initial U.S. Approval: 1997
RECENT MAJOR CHANGES
245.25.4 INDICATIONS AND USAGE
-
● Tamsulosin hydrochloride capsules is an alpha1 adrenoceptor antagonist indicated for treatment of the signs and symptoms of benign prostatic hyperplasia (1)
-
● Tamsulosin hydrochloride capsules are not indicated for the treatment of hypertension (1)
-
● 0.4 mg once daily taken approximately one-half hour following the same meal each day (2)
-
● Can be increased to 0.8 mg once daily for patients who fail to respond to the 0.4 mg dose after 2 to 4 weeks of dosing (2)
-
● If discontinued or interrupted for several days, therapy should start again with the 0.4 mg once-daily dose (2)
-
● Capsules: 0.4 mg (3)
-
● Contraindicated in patients known to be hypersensitive to tamsulosin hydrochloride or any component of tamsulosin hydrochloride capsules (4,6.2)
-
● Advise patients about the possibility of symptoms related to postural hypotension and to avoid situations where injury could result should syncope occur (5.1)
-
● Should not be used in combination with strong inhibitors of CYP3A4 (5.2,7.1). Use with caution in combination with moderate inhibitors of CYP3A4, with strong or moderate inhibitors of CYP2D6, in patients known to be CYP2D6 poor metabolizers, or in combination with other cytochrome P450 inhibitors. (5.2,7.1,12.3)
-
● Should not be used in combination with other alpha adrenergic blocking agents (5.2,7.2,12.3)
-
● Exercise caution with concomitant administration of warfarin (5.2,7.4,12.3)
-
● Advise patients about the possibility and seriousness of priapism (5.3)
-
● Intraoperative Floppy Iris Syndrome has been observed during cataract surgery in some patients. Advise patients considering cataract surgery to tell their ophthalmologist that they have taken tamsulosin hydrochloride capsules. (5.5)
-
● Advise patients to be screened for the presence of prostate cancer prior to treatment and at regular intervals afterwards (5.4)
-
● The most common adverse events (of patients and at a higher incidence than placebo) with the 0.4 mg dose or 0.8 mg dose were headache, dizziness, rhinitis, infection, abnormal ejaculation, asthenia, back pain, diarrhea, pharyngitis, chest pain, cough increased, somnolence, nausea, sinusitis, insomnia, libido decreased, tooth disorder, and blurred vision (6.1)
DRUG INTERACTIONS
-
● Tamsulosin hydrochloride capsules 0.4 mg should not be used with strong inhibitors of CYP3A4 (e.g., ketoconazole). Tamsulosin hydrochloride capsules should be used with caution in combination with moderate inhibitors of CYP3A4 (e.g., erythomycin), in combination with strong (e.g.,paroxetine) or moderate (e.g.,terbinafine) inhibitors of CYP2D6, or in patients known to be CYP2D6 poor metabolizers, particularly at a dose higher than 0.4 mg (e.g., 0.8 mg) (5.2,7.1,12.3)
-
● Concomitant use of PDE5 inhibitors with tamsulosin can potentially cause symptomatic hypotension (5.2,7.3,12.3)
-
● Pediatric Use: Not indicated for use in pediatric populations (8.4,12.3)
-
● Geriatric Use: No overall differences in efficacy or safety vs. younger patients, but greater sensitivity of some older adults cannot be ruled out (8.5,12.3)
-
● Renal Impairment: Has not been studied in patients with end stage renal disease (8.6,12.3)
-
● Hepatic Impairment: Has not been studied in patients with severe hepatic impairment (8.7,12.3)
See17for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling
*
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1 Orthostasis
5.2 Drug Interactions
5.3 Priapism
5.4 Screening for Prostate Cancer
5.5 Intraoperative Floppy Iris Syndrome
5.6 Sulfa Allergy
6. ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7. DRUG INTERACTIONS
7.1 Cytochrome P450 Inhibition
7.2 Other Alpha Adrenergic Blocking Agents
7.3 PDE5 Inhibitors
7.4 Warfarin
7.5 Nifedipine, Atenolol, Enalapril
7.6 Digoxin and Theophylline
7.7 Furosemide
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10. OVERDOSAGE
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14. CLINICAL STUDIES
16. HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Hypotension
17.2 Priapism
17.3 Screening for Prostate Cancer
17.4 Intraoperative Floppy Iris Syndrome
17.5 Administration
17.6 FDA-approved Patient Labeling
PRINCIPAL DISPLAY PANEL - 0.4 mg Capsule Bottle Label
*
1.0 INDICATIONS & USAGE
Clinical Studies (14)2.0 DOSAGE & ADMINISTRATION
Warnings and Precautions (5.2)
3.0 DOSAGE FORMS & STRENGTHS
4.0 CONTRAINDICATIONS
Adverse Reactions (6.2)5.0 WARNINGS
5.1 OrthostasisAdverse Reactions (6.1)Patient Counseling Information (17.1)
5.2 Drug Interactions
Drug Interactions (7.1)Clinical Pharmacology (12.3)Drug Interactions (7.1)Clinical Pharmacology (12.3)
Drug Interactions (7.1)Clinical Pharmacology (12.3)
Drug Interactions (7.2)Clinical Pharmacology (12.3)
Drug Interactions (7.3)Clinical Pharmacology (12.3)
Drug Interactions (7.4)Clinical Pharmacology (12.3)
5.3 Priapism
Patient Counseling Information (17.2)
5.4 Screening for Prostate Cancer
Patient Counseling Information (17.3)
5.5 Intraoperative Floppy Iris Syndrome
Adverse Reactions (6.2)
5.6 Sulfa Allergy
6.0 ADVERSE REACTIONS
6.1 Clinical Trials ExperienceTable 1
*
BODY SYSTEM/TAMSULOSIN HYDROCHLORIDEPLACEBOCAPSULES GROUPSADVERSE EVENT0.4 mg n=5020.8 mg n=492n=493BODY AS WHOLENERVOUS SYSTEMRESPIRATORY SYSTEMDIGESTIVE SYSTEMUROGENITAL SYSTEM*
-
● The adverse event occurred for the first time after initial dosing with double-blind study medication.
-
● The adverse event was present prior to or at the time of initial dosing with double-blind study medication and subsequently increased in severity during double-blind treatment; or
-
● The adverse event was present prior to or at the time of initial dosing with double-blind study medication, disappeared completely, and then reappeared during double-blind treatment.
Warnings and Precautions (5.1)
6.2 Postmarketing Experience
Warnings and Precautions (5.5)
7.0 DRUG INTERACTIONS
7.1 Cytochrome P450 InhibitionWarnings and Precautions (5.2)Clinical Pharmacology (12.3)Warnings and Precautions (5.2)Clinical Pharmacology (12.3)
Warnings and Precautions (5.2)Clinical Pharmacology (12.3)Warnings and Precautions (5.2)Clinical Pharmacology (12.3)
Warnings and Precautions (5.2)Clinical Pharmacology (12.3)
Warnings and Precautions (5.2)Clinical Pharmacology (12.3)
Warnings and Precautions (5.2)Clinical Pharmacology (12.3)
7.2 Other Alpha Adrenergic Blocking Agents
Warnings and Precautions (5.2)Clinical Pharmacology (12.3)
7.3 PDE5 Inhibitors
Warnings and Precautions (5.2)Clinical Pharmacology (12.3)
7.4 Warfarin
Warnings and Precautions (5.2)Clinical Pharmacology (12.3)
7.5 Nifedipine, Atenolol, Enalapril
Warnings and PrecautionsClinical Pharmacology (12.3)
7.6 Digoxin and Theophylline
Clinical Pharmacology (12.3)
7.7 Furosemide
Warnings and PrecautionsClinical Pharmacology (12.3)
8.0 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
Clinical Pharmacology (12.3)
8.6 Renal Impairment
Clinical Pharmacology (12.3)
8.7 Hepatic Impairment
Clinical Pharmacology (12.3)
9.0 OVERDOSAGE
Warnings and Precautions (5.1) Adverse Reactions (6.1)10.0 DESCRIPTION
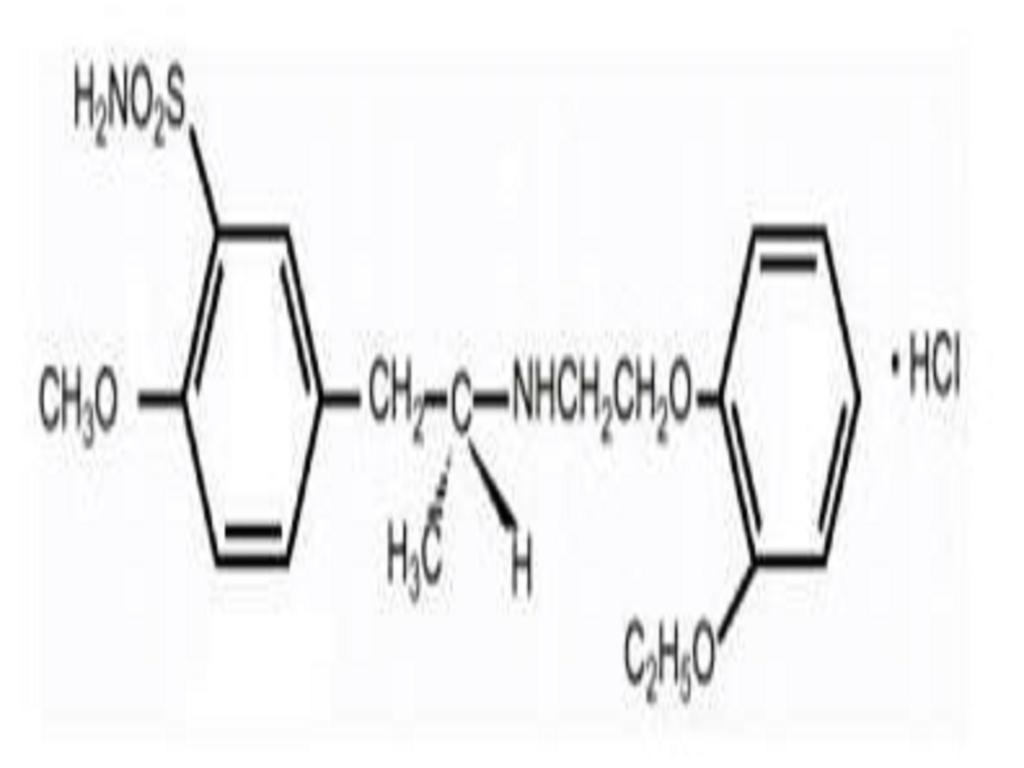
12.0 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action12.2 Pharmacodynamics
Use in Specific Population (8.4)Clinical Studies (14)
12.3 Pharmacokinetics
Figure 1
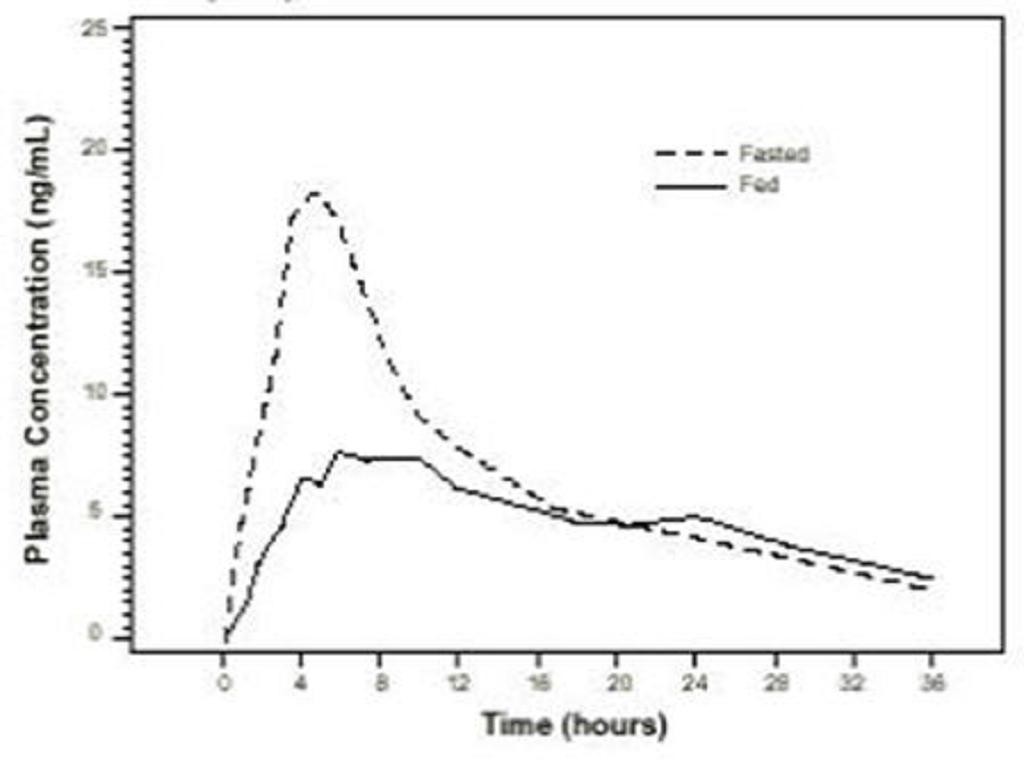
Table 2
Pharma-cokineticn=23 (age range 18 to 32 years)n=22 (age range 55 to 75 years)ParameterLight BreakfastFastedLightHigh-FatFastedBreakfastBreakfast
Warnings and Precautions (5.2)Drug Interactions (7.1)
Use in Specific Populations (8.4)
Use in Specific Populations (8.5)
Use in Specific Populations (8.6)
Use in Specific Populations (8.7)
Warnings and Precautions (5.2)Clinical Pharmacology (12.3)Warnings and Precautions (5.2)Drug Interactions (7.1)
Warnings and Precautions (5.2)Drug Interactions (7.1)Warnings and Precautions (5.2)Drug Interactions (7.1)
Warnings and Precautions (5.2)Drug Interactions (7.1)
Warnings and Precautions (5.2)Drug Interactions (7.1)
Warnings and Precautions (5.2)Drug Interactions (7.1)
Warnings and Precautions (5.2)Drug Interactions (7.2)
Warnings and Precautions (5.2)Drug Interactions (7.3)
Warnings and Precautions (5.2)Drug Interactions (7.4)
Drug Interactions (7.5)
Drug Interactions (7.6)
Drug Interactions (7.7)
13.0 CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility14.0 CLINICAL STUDIES
Table 3Figures 2A2BTable 3Figures 3A3B
*
Total AUA Symptom ScorePeak Urine Flow RateMean Base-Mean ChangeMean Base-Mean Changeline Valueline ValueStudy 2
*
Figures 2A2B
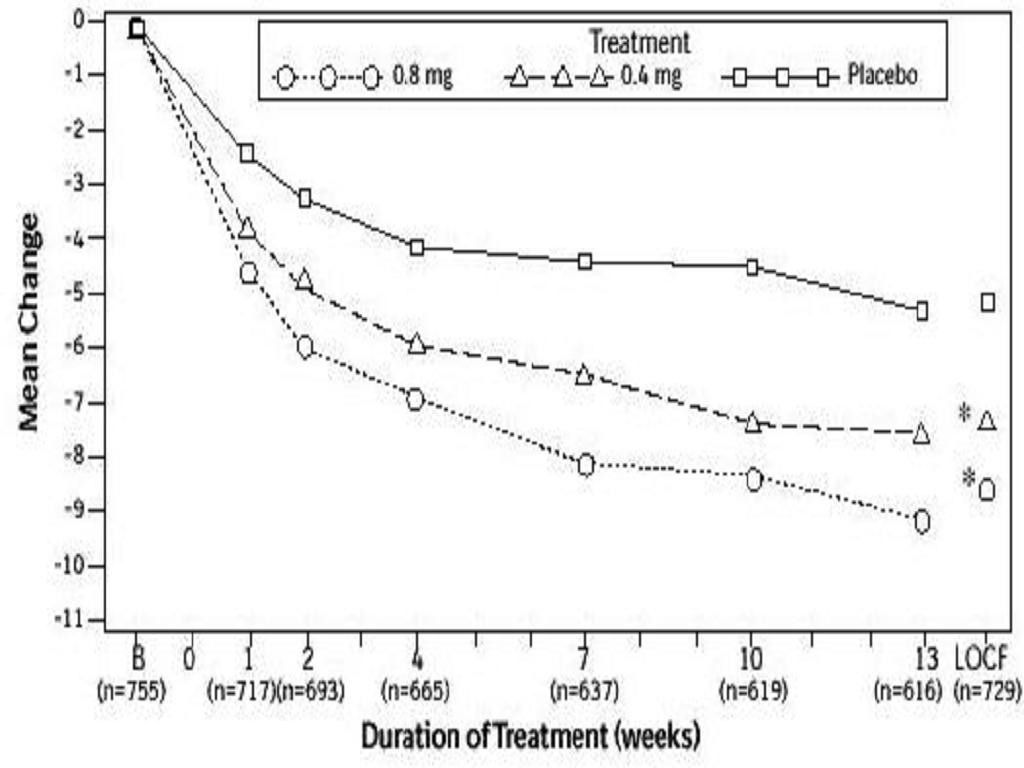

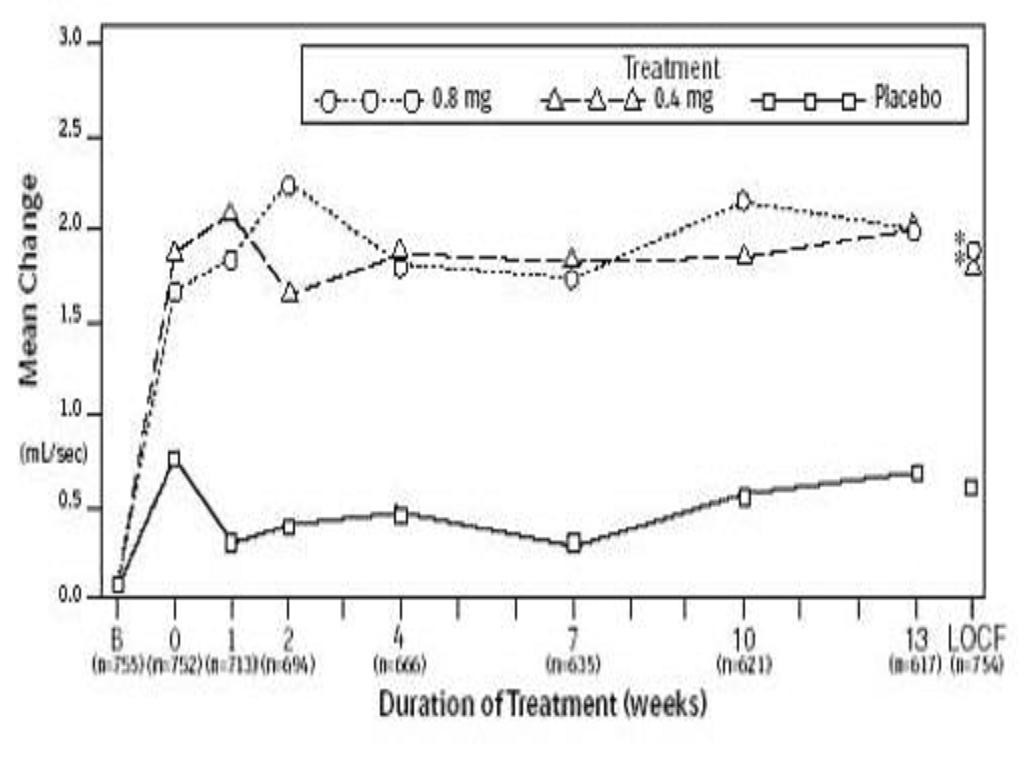

15.0 HOW SUPPLIED
17.0 SPL PATIENT PACKAGE INSERT
PATIENT COUNSELING INFORMATIONFDA-Approved Patient Labeling (17.6)
17.1 Hypotension
Warnings and Precautions (5.1)
17.2 Priapism
Warnings and Precautions (5.3)
17.3 Screening for Prostate Cancer
Warnings and Precautions (5.4)
17.4 Intraoperative Floppy Iris Syndrome
Warnings and Precautions (5.5)
17.5 Administration
17.6 FDA-approved Patient Labeling
INFORMATION FOR PATIENTS
-
● Tamsulosin hydrochloride capsules are not for women.
-
● Tamsulosin hydrochloride capsules are not for children.
ingredients
-
● any kidney or liver problems.
-
● any history of low blood pressure.
-
● any allergies to sulfa or any other medicines.
-
● if you are planning to have cataract surgery.
-
● any prescription medicines, including blood pressure medicines.
-
● any non-prescription medicines, including vitamins and herbal supplements.
-
● Take tamsulosin hydrochloride capsules exactly as prescribed by your doctor.
-
● Do not crush, chew, or open tamsulosin hydrochloride capsules.
-
● Take tamsulosin hydrochloride capsules one time each day, about 30 minutes after the same meal each day. For example, you may take tamsulosin hydrochloride capsules 30 minutes after dinner each day.
-
● If you miss a dose of tamsulosin hydrochloride capsules, take it as soon as you remember. If you miss your dose for the whole day, continue with your next dose on your regular schedule. Do not take two doses at the same time.
-
● If you stop or forget to take tamsulosin hydrochloride capsules for several days, talk with your doctor before starting again.
-
● If you take more tamsulosin hydrochloride capsules than prescribed, call your doctor right away.
-
● Decreased blood pressure when changing positions. Tamsulosin hydrochloride capsules may cause a sudden drop in blood pressure upon standing, especially after the first dose or when changing doses.
-
● fainting
-
● dizziness
-
● lightheadedness
-
● Allergic reactions. Make your doctor aware of any allergic reactions you may experience while taking tamsulosin hydrochloride capsules.
-
● rash
-
● itching
-
● hives
-
● swelling of face, tongue, or throat
-
● difficulty breathing
-
● A painful erection that will not go away. Tamsulosin hydrochloride capsules can cause a painful erection (priapism), which cannot be relieved by having sex. If this happens, get medical help right away. If priapism is not treated, you may not be able to get an erection in the future.
-
● Eye problems during cataract surgery. During cataract surgery, a condition called intraoperative floppy iris syndrome (IFIS) can happen if you take or have taken tamsulosin hydrochloride capsules. If you need to have cataract surgery, be sure to tell your surgeon if you take or have taken tamsulosin hydrochloride capsules.
-
● runny nose
-
● dizziness
-
● decreased semen
What are the side effects of tamsulosin hydrochloride capsules
-
● Active Ingredient: tamsulosin hydrochloride
-
● Inactive Ingredients: methacrylic acid copolymer, sugar spheres, dibutyl sebacate, magnesium stearate, talc, ammonio methacrylate copolymer, copovidone, colloidal silicon dioxide, and hard gelatin capsule. Sugar spheres contain sucrose and starch. Gelatin capsules contain edible ink, titanium dioxide, gelatin, D&C red No. 28, D&C yellow No. 10, FD&C red No. 40, and FD&C green No. 3. The capsule imprinting ink Black SW-9008-/SW-9009 contains strong ammonia solution, black iron oxide, potassium hydroxide, propylene glycol, and shellac.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


Tamsulosin HydrochlorideTamsulosin Hydrochloride CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!