Sulfamethoxazole and Trimethoprim
FULL PRESCRIBING INFORMATION: CONTENTS*
- SULFAMETHOXAZOLE AND TRIMETHOPRIM DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- SULFAMETHOXAZOLE AND TRIMETHOPRIM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- SULFAMETHOXAZOLE AND TRIMETHOPRIM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
SULFAMETHOXAZOLE AND TRIMETHOPRIM DESCRIPTION
DESCRIPTION
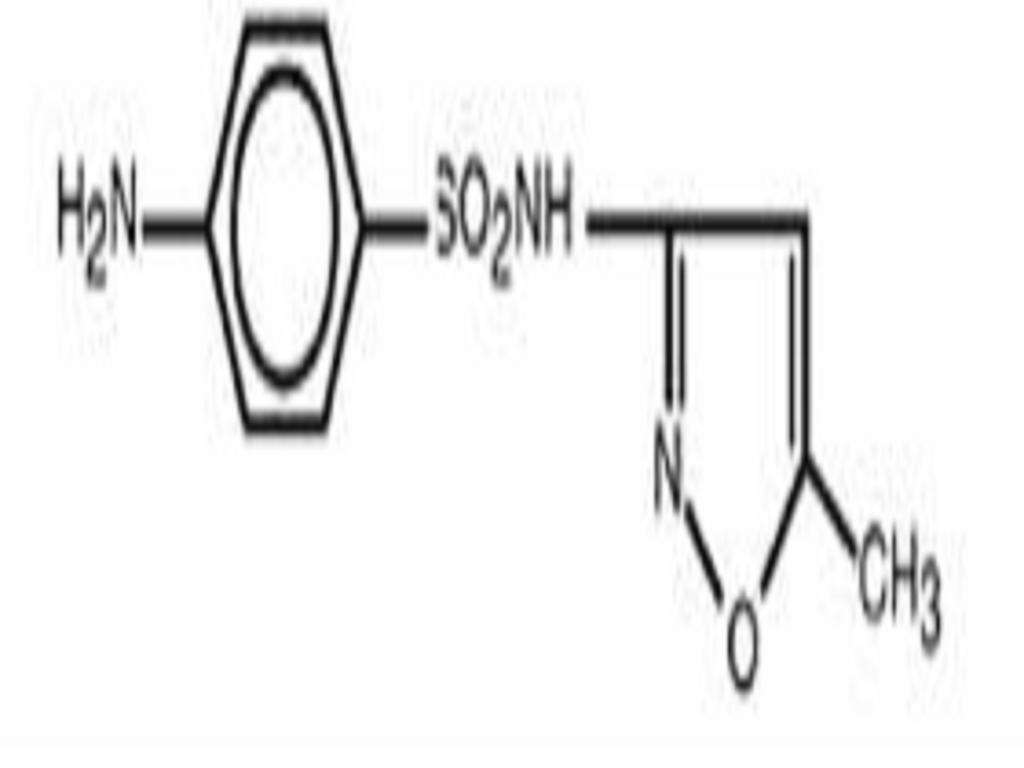
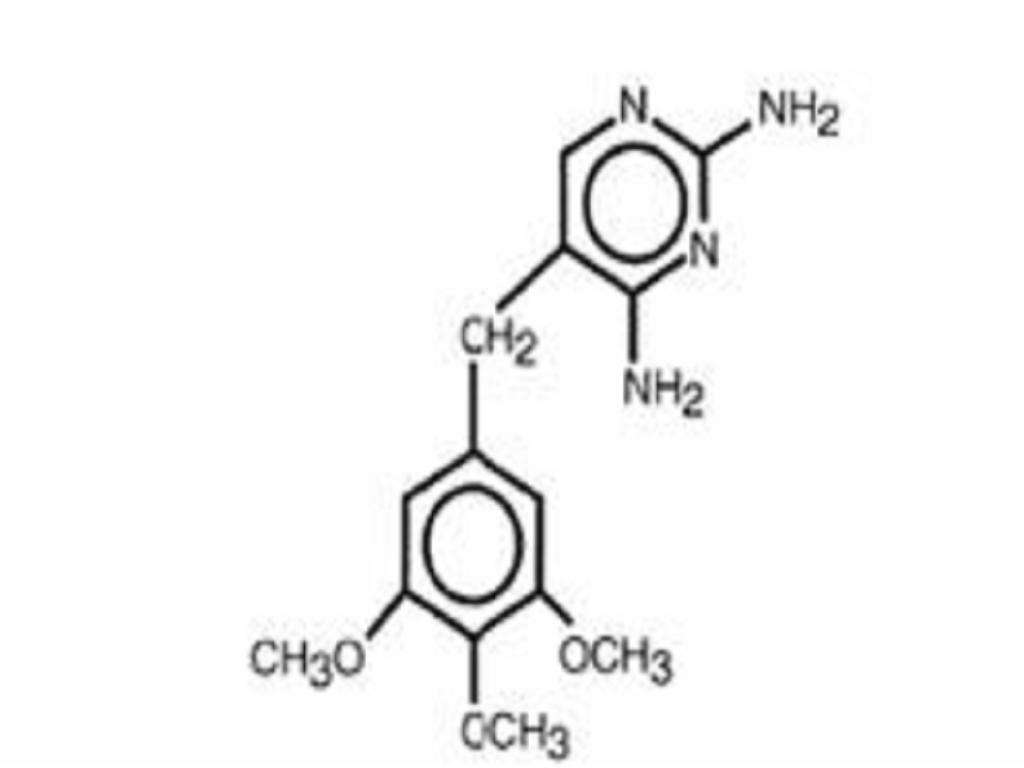
CLINICAL PHARMACOLOGY
DOSAGE AND ADMINISTRATION
INDICATIONS & USAGE
SULFAMETHOXAZOLE AND TRIMETHOPRIM CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
PRECAUTIONS
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
WARNINGS
INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
CONTRAINDICATIONS
NURSING MOTHERS
CONTRAINDICATIONSPEDIATRIC USE
INDICATIONSCONTRAINDICATIONSGERIATRIC USE
WARNINGSADVERSE REACTIONSDOSAGE AND ADMINISTRATION
CLINICAL PHARMACOLOGY: Geriatric Pharmacokinetics
SULFAMETHOXAZOLE AND TRIMETHOPRIM ADVERSE REACTIONS
WARNINGSPRECAUTIONS: Use in the Treatment of and Prophylaxis for Pneumocystis Carinii Pneumonia in Patients with Acquired Immunodeficiency Syndrome (AIDS
WARNINGS
OVERDOSAGE
DOSAGE & ADMINISTRATION
HOW SUPPLIED
REFERENCES
INACTIVE INGREDIENT
POVIDONESODIUMSTARCH GLYCOLATE TYPE A POTATO
MAGNESIUM STEARATE
STARCH, CORN
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Sulfamethoxazole and TrimethoprimSulfamethoxazole and Trimethoprim TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!