Divalproex Sodium Extended Release
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- DOSAGE FORMS & STRENGTHS
- DIVALPROEX SODIUM EXTENDED RELEASE CONTRAINDICATIONS
- WARNINGS AND PRECAUTIONS
- DIVALPROEX SODIUM EXTENDED RELEASE ADVERSE REACTIONS
- DRUG INTERACTIONS
- USE IN SPECIFIC POPULATIONS
- OVERDOSAGE
- DIVALPROEX SODIUM EXTENDED RELEASE DESCRIPTION
- CLINICAL PHARMACOLOGY
- NONCLINICAL TOXICOLOGY
- CLINICAL STUDIES
- HOW SUPPLIED
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
BOXED WARNINGWarnings and Precautions (5.1)
Warnings and Precautions (5.2)
Patient Counseling Information (17.8)
Warnings and Precautions (5.3)
INDICATIONS & USAGE
1 INDICATIONS AND USAGE1.1 Mania
Clinical Studies (14.1)
1.2 Epilepsy
1.3 Migraine
Warnings and Precautions (5.2)Patient Counseling Information (17.3)
DOSAGE & ADMINISTRATION
2 DOSAGE AND ADMINISTRATION2.1 Mania
2.2 Epilepsy
Drug Interactions (7.2)
Complex Partial Seizures
Monotherapy (Initial Therapy)
Conversion to Monotherapy
Adjunctive Therapy
Clinical Studies (14.3)Drug Interactions (7)
Simple and Complex Absence Seizures
Clinical Pharmacology (12.3)
Drug Interactions (7.2)
2.3 Migraine
2.4 Conversion from Divalproex Sodium Delayed-Release Tablets to Divalproex Sodium Extended-Release Tablets
Table 1 Dose Conversion
Divalproex Sodium Delayed-Release TabletsDivalproex Sodium Extended-Release Tablets****
Clinical Pharmacology (12.2)
2.5 General Dosing Advice
Dosing in Elderly Patients
Warnings and Precautions (5.12)
Dose Related Adverse Reactions
Warnings and Precautions (5.6)
G.I. Irritation
Compliance
DOSAGE FORMS & STRENGTHS
3 DOSAGE FORMS AND STRENGTHSDIVALPROEX SODIUM EXTENDED RELEASE CONTRAINDICATIONS
4 CONTRAINDICATIONS-
? Divalproex sodium extended-release tablets should not be administered to patients with hepatic disease or significant hepatic dysfunction [seeWarnings and Precautions (5.1)].
-
? Divalproex sodium extended-release tablets are contraindicated in patients with known hypersensitivity to the drug [seeWarnings and Precautions (5.10)].
-
? Divalproex sodium extended-release tablets are contraindicated in patients with known urea cycle disorders [seeWarnings and Precautions (5.4)].
-
?
WARNINGS AND PRECAUTIONS
5 WARNINGS AND PRECAUTIONS5.1 Hepatotoxicity
Boxed WarningContraindications (4)
5.2 Teratogenicity/Usage in Pregnancy
Boxed WarningUse in Specific Populations (8.1)
5.3 Pancreatitis
Boxed Warning
5.4 Urea Cycle Disorders
Contraindications (4) Warnings and Precautions (5.7)
5.5 Suicidal Behavior and Ideation
Table 2. Risk by Indication for Antiepileptic Drugs in the Pooled Analysis
5.6 Thrombocytopenia
5.7 Hyperammonemia
Warnings and Precautions (5.9)Contraindications (4)Warnings and Precautions (5.45.8)
5.8 Hyperammonemia and Encephalopathy Associated with Concomitant Topiramate Use
Warnings and Precautions (5.9)Contraindications (4)Warnings and Precautions (5.7)
5.9 Hypothermia
Drug Interactions (7.3)
5.10 Multi-Organ Hypersensitivity Reactions
5.11 Interaction with Carbapenem Antibiotics
Drug Interactions (7.1)
5.12 Somnolence in the Elderly
Dosage and Administration (2.4)
5.13 Monitoring: Drug Plasma Concentration
Drug Interactions (7)
5.14 Effect on Ketone and Thyroid Function Tests
5.15 Effect on HIV and CMV Viruses Replication
DIVALPROEX SODIUM EXTENDED RELEASE ADVERSE REACTIONS
6 ADVERSE REACTIONS6.1 Mania
*
Adverse EventDivalproex Sodium Extended-ReleasePlaceboTablets(N = 263)(N = 338)*
6.2 Epilepsy
Body System/EventDivalproex Sodium Delayed-Placebo (%)Release Tablets (%)(N = 70)(N = 77)Body as a WholeGastrointestinal SystemNervous SystemRespiratory SystemOther
*
Body System/EventHigh Dose (%)Low Dose (%)(N = 131)(N = 134)Body as a WholeDigestive SystemHemic/Lymphatic SystemMetabolic/NutritionalNervous SystemRespiratory SystemSkin and AppendagesAlopecia*
6.3 Migraine
*
Body System EventDivalproex Sodium Extended-releasePlaceboTablets(N = 115)(N = 122)Gastrointestinal SystemNervous SystemOther*
*
Body System ReactionDivalproex SodiumPlaceboDelayed-release Tablets(N = 81)(N = 202)Gastrointestinal SystemNervous SystemOther*
6.4 Other Patient Populations
Mania
Epilepsy
Gastrointestinal
CNS Effects
Warnings and Precautions (5.4)
Dermatologic
Drug Interactions (7)
Psychiatric
Musculoskeletal
Hematologic
Warnings and Precautions (5.6)Drug Interactions (7)
Hepatic
Warnings and Precautions (5.1)
Endocrine
Warnings and Precautions (5.13)
Pancreatic
Warnings and Precautions (5.3)
Metabolic
Warnings and Precautions (5.7)
Genitourinary
Special Senses
Other
DRUG INTERACTIONS
7 DRUG INTERACTIONS7.1 Effects of Coadministered Drugs on Valproate Clearance
Drugs for Which a Potentially Important Interaction has Been Observed
Aspirin
Carbapenem Antibiotics
Warnings and Precautions (5.11)
Felbamate
Rifampin
Drugs for Which Either no Interaction or a Likely Clinically Unimportant Interaction has Been Observed
Antacids
Chlorpromazine
Haloperidol
Cimetidine and Ranitidine
7.2 Effects of Valproate on Other Drugs
Drugs for Which a Potentially Important Valproate Interaction has Been Observed
Amitriptyline/Nortriptyline
Carbamazepine/carbamazepine-10,11-Epoxide
Clonazepam
Diazepam
Ethosuximide
Lamotrigine
Phenobarbital
Phenytoin
Tolbutamide
Warfarin
Zidovudine
Drugs for Which Either no Interaction or a Likely Clinically Unimportant Interaction has Been Observed
Acetaminophen
Clozapine
Lithium
Lorazepam
Oral Contraceptive Steroids
7.3 Topiramate
Contraindications (4)Warnings and Precautions (5.75.8)Warnings and Precautions (5.75.9)
USE IN SPECIFIC POPULATIONS
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy
Human Data
Neural Tube Defects
Other Adverse Pregnancy Effects
Warnings and Precautions (5.6)
Warnings and Precautions (5.1)
Animal Data
Registry
8.3 Nursing Mothers
8.4 Pediatric Use
Mania
Migraine Prophylaxis
Epilepsy
Pediatric Safety
Safety Studies-Mania
Safety Study-Controlled Mania Trial
Adverse Reaction- Preferred TermDivalproex Sodium Extended-release Tablets (N = 76)Placebo (N = 74)
Safety Study-Open-Label Mania Safety Data
Adverse Reactions (6.16.26.3)
Safety Studies-Epilepsy (Open-Label)
Safety Studies-Migraine (Controlled and Open-Label)
Prior Safety Experience
Boxed WarningWarnings and Precautions (5.1)
Nonclinical Developmental Toxicology
8.5 Geriatric Use
Warnings and Precautions (5.12)Dosage and Administration (2.4)
Clinical Pharmacology (12.3)
8.6 Effect of Disease
Liver Disease
Boxed WarningContraindications (4)Warnings and Precautions (5)Clinical Pharmacology (12.3)
OVERDOSAGE
10 OVERDOSAGEDIVALPROEX SODIUM EXTENDED RELEASE DESCRIPTION
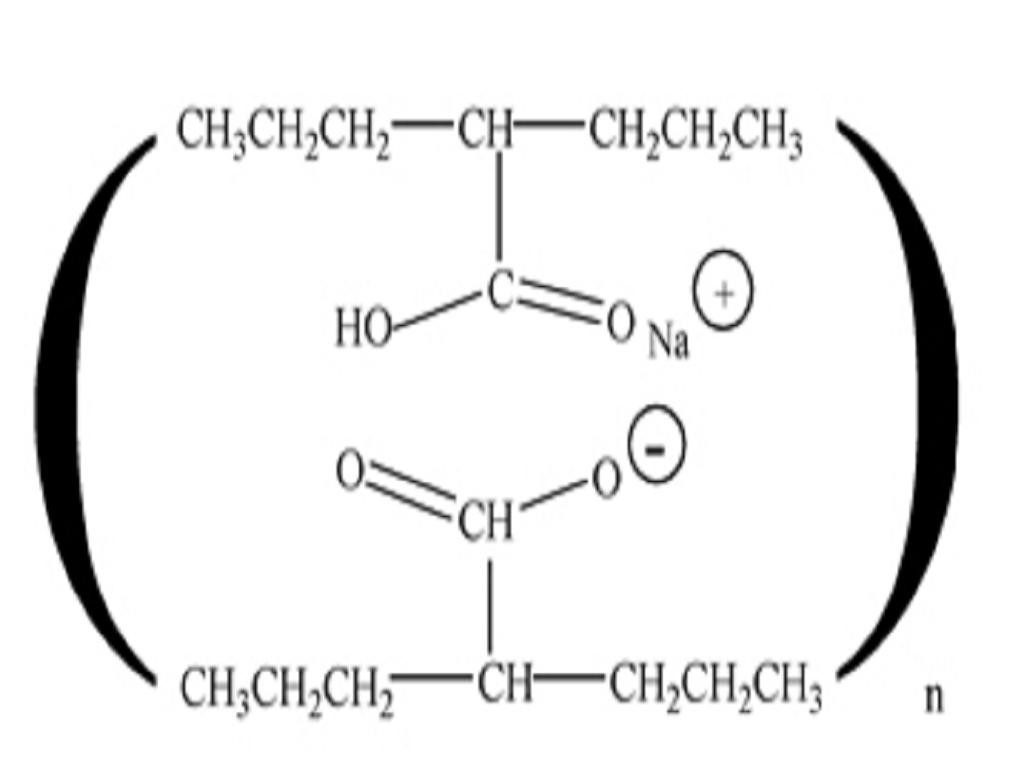
CLINICAL PHARMACOLOGY
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action
12.2 Pharmacodynamics
Epilepsy
Mania
Dosage and Administration (2.1)
12.3 Pharmacokinetics
Absorption/Bioavailability
Conversion from Divalproex Sodium Delayed-Release Tablets to Divalproex Sodium Extended-Release Tablets
Table 9 Bioavailability of Divalproex Sodium Extended-Release Tablets Relative to Divalproex Sodium Delayed-Release Tablets When Divalproex Sodium Extended-Release Tablets Dose is 8% to 20% Higher
Study PopulationRegimensRelativeBioavailablility
Distribution
Protein Binding
Drug Interactions (7)
CNS Distribution
Metabolism
Elimination
Special Populations
Effect of Age
Pediatric
Elderly
Dosage and Administration (2.4)
Effect of Sex
Effect of Race
Effect of Disease
Liver Disease
Boxed WarningContraindications (4)Warnings and Precautions (5.1)
Renal Disease
NONCLINICAL TOXICOLOGY
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Mutagenesis
Fertility
CLINICAL STUDIES
14 CLINICAL STUDIES14.1 Mania
14.2 Epilepsy
**
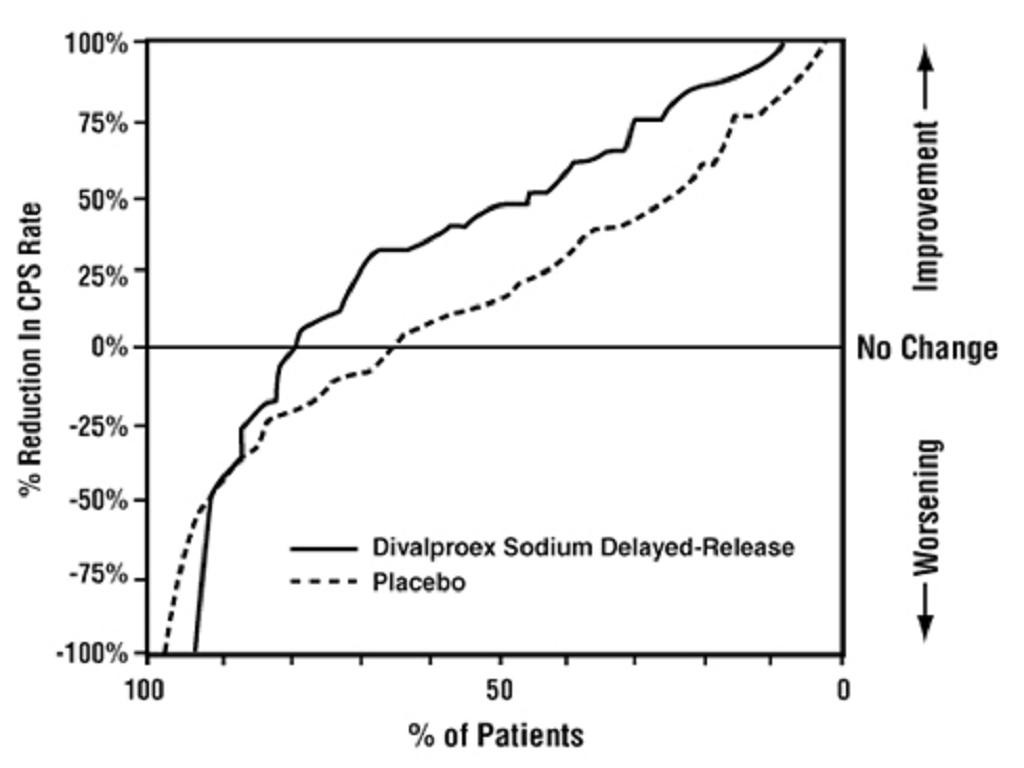
**
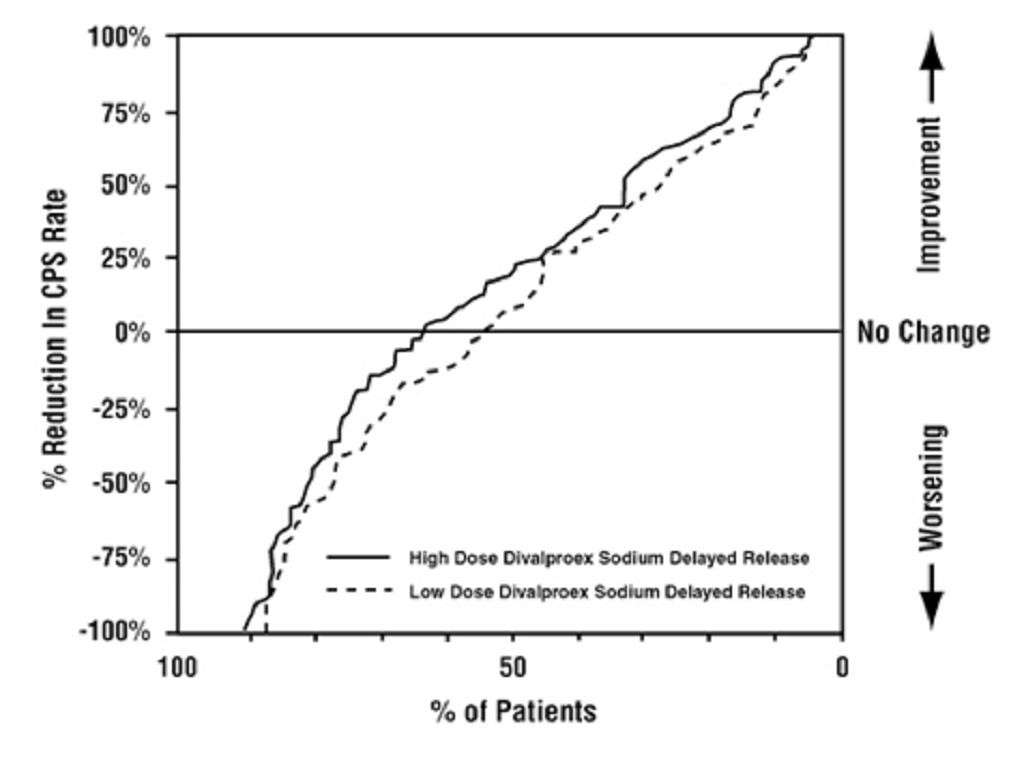
14.3 Migraine
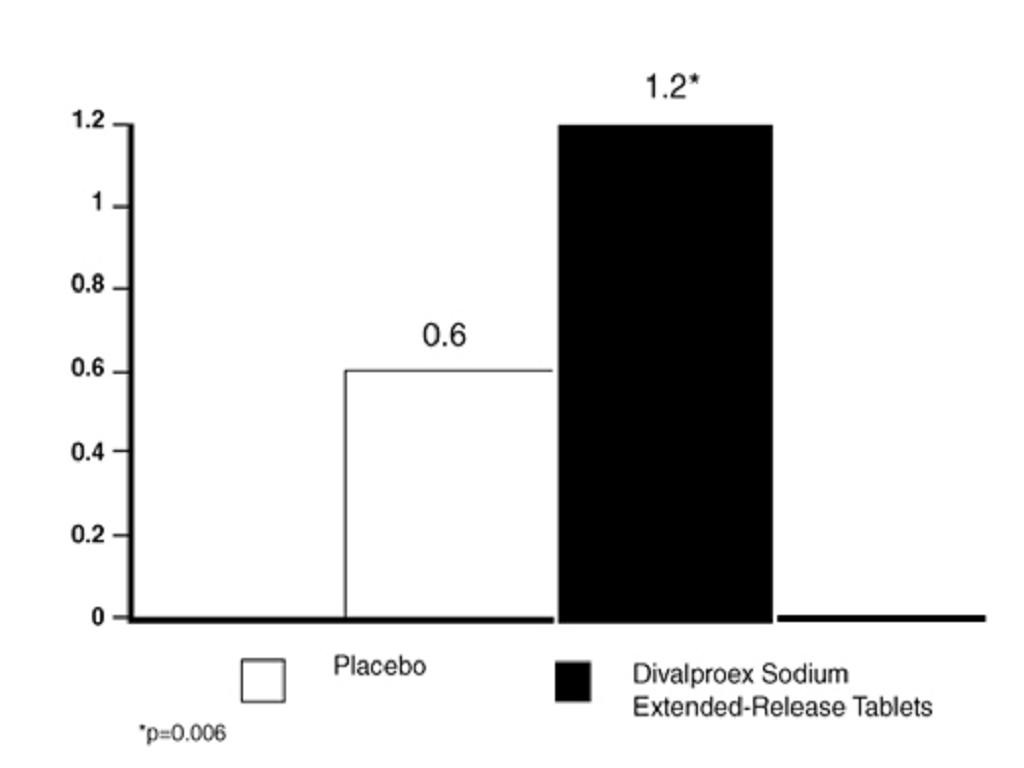
HOW SUPPLIED
16 HOW SUPPLIED/STORAGE AND HANDLINGINFORMATION FOR PATIENTS
17 PATIENT COUNSELING INFORMATIONFDA-Approved Patient Labeling (17.8)
17.1 Hepatotoxicity
Warnings and Precautions (5.1)
17.2 Pancreatitis
Warnings and Precautions (5.3)
17.3 Teratogenicity/Usage in Pregnancy
Use in Specific Populations (8.1)
Use in Specific Populations (8.1)
17.4 Suicidal Thinking and Behavior
Warnings and Precautions (5.5)
17.5 Hyperammonemia
Warnings and Precautions (5.75.8)
17.6 CNS Depression
17.7 Multi-Organ Hypersensitivity Reaction
Warnings and Precautions (5.10)
17.8 FDA-Approved Patient Labeling
-
? You should take your medicine exactly as prescribed by your doctor to get the most benefit from your medicine and reduce the risk of side effects.
-
? If you have taken more than the prescribed dose, contact your hospital emergency room or local poison center immediately.
-
? Your medicine was prescribed for your particular condition. Do not use it for another condition or give the drug to others.
-
? Facts About Birth Defects
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Divalproex Sodium Extended ReleaseDivalproex Sodium Extended Release TABLET, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!