Diclofenac Sodium
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- DICLOFENAC SODIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- INDICATIONS & USAGE
- DICLOFENAC SODIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- DICLOFENAC SODIUM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Cardiovascular Risk-
● NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk (seeWARNINGS).
-
● Diclofenac sodium delayed-release tablets are contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (seeWARNINGS).
-
● NSAIDs cause an increased risk of serious gastrointestinal adverse events including inflammation, bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events (seeWARNINGS).
DICLOFENAC SODIUM DESCRIPTION
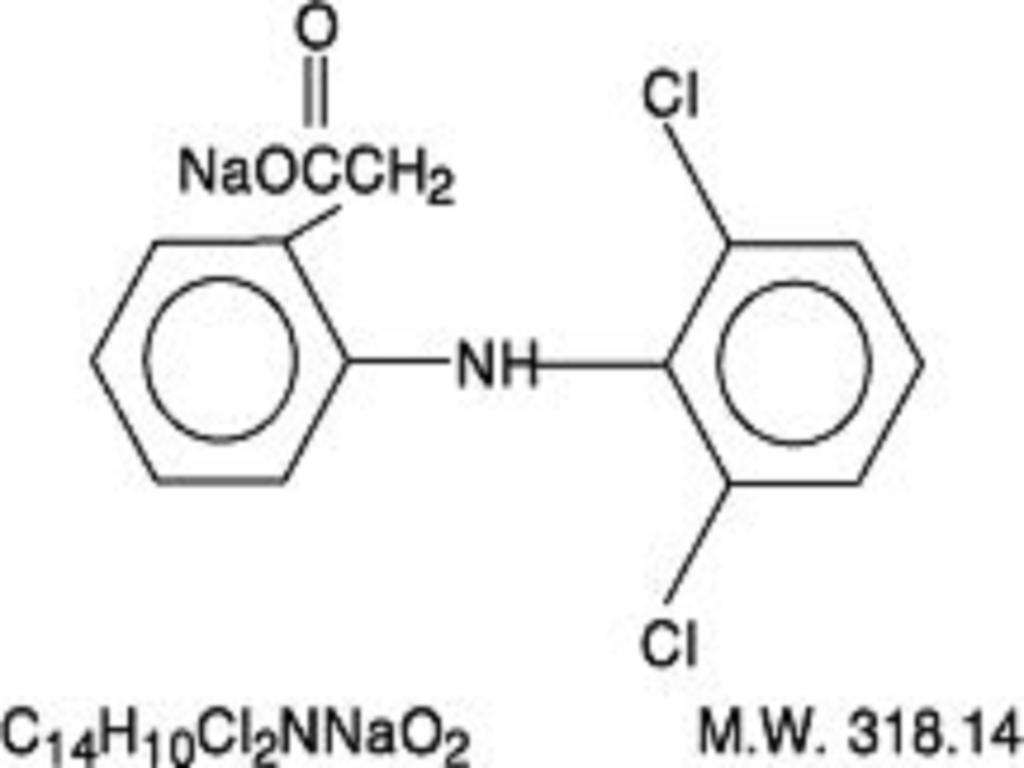
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS
PHARMACOKINETICS
AbsorptionTable 1
Distribution
Metabolism
Excretion
Special Populations
Pediatric
Race
Hepatic Insufficiency
Renal Insufficiency
INDICATIONS & USAGE
WARNINGS-
● For relief of the signs and symptoms of osteoarthritis
-
● For relief of the signs and symptoms of rheumatoid arthritis
-
● For acute or long-term use in the relief of signs and symptoms of ankylosing spondylitis
DICLOFENAC SODIUM CONTRAINDICATIONS
WARNINGS: Anaphylactoid ReactionsPRECAUTIONS: Preexisting Asthma
WARNINGS
WARNINGS
Cardiovascular EffectsCardiovascular Thrombotic Events
WARNINGS: Gastrointestinal (GI) Effects
CONTRAINDICATIONS
Hypertension
Congestive Heart Failure and Edema Renal Effects
Gastrointestinal (GI) Effects
Risk of GI Ulceration, Bleeding, and Perforation
Renal Effects
Advanced Renal Disease
Hepatic Effects
Anaphylactoid Reactions
CONTRAINDICATIONSPRECAUTIONS: Preexisting Asthma
Skin Reactions
Pregnancy
PRECAUTIONS
GeneralHematological Effects
Preexisting Asthma
INFORMATION FOR PATIENTS
WARNINGS: Cardiovascular Effects
WARNINGS: Gastrointestinal (GI) Effects: Risk of GI Ulceration, Bleeding, and Perforation
WARNINGS: Hepatic Effects
WARNINGS
LABORATORY TESTS
DRUG INTERACTIONS
AspirinMethotrexate
Cyclosporine
ACE-inhibitors
Furosemide
WARNINGS: Renal Effects
Lithium
Warfarin
PREGNANCY
Teratogenic EffectsPregnancy Category C
Nonteratogenic Effects
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
DICLOFENAC SODIUM ADVERSE REACTIONS
Body as a Whole
Cardiovascular System
Digestive System
Hemic and Lymphatic System
Metabolic and Nutritional
Nervous System
Respiratory System
Skin and Appendages
Special Senses
Urogenital System
Body as a Whole
Cardiovascular System
Digestive System
Hemic and Lymphatic System
Metabolic and Nutritional
Nervous System
Respiratory System
Skin and Appendages
Special Senses
OVERDOSAGE
DOSAGE & ADMINISTRATION
WARNINGSHOW SUPPLIED
SPL MEDGUIDE
for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)-
● with longer use of NSAID medicines
-
● in people who have heart disease
-
● can happen without warning symptoms
-
● may cause death
-
● taking medicines calledcorticosteroidsandanticoagulants
-
● longer use
-
● smoking
-
● drinking alcohol
-
● older age
-
● having poor health
-
● exactly as prescribed
-
● at the lowest dose possible for your treatment
-
● for the shortest time needed
-
● different types of arthritis
-
● menstrual cramps and other types of short-term pain
-
● if you had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAID medicine
-
● for pain right before or after heart bypass surgery
-
● about all your medical conditions.
-
● about all of the medicines you take. NSAIDs and some other medicines can interact with each other and cause serious side effects. Keep a list of your medicines to show to your healthcare provider and pharmacist.
-
● if you are pregnant. NSAID medicines should not be used by pregnant women late in their pregnancy.
-
● if you are breastfeeding. Talk to your doctor.
-
● shortness of breath or trouble breathing
-
● chest pain
-
● weakness in one part or side of your body
-
● slurred speech
-
● swelling of the face or throat
-
● nausea
-
● more tired or weaker than usual
-
● itching
-
● your skin or eyes look yellow
-
● stomach pain
-
● flu-like symptoms
-
● vomit blood
-
● there is blood in your bowel movement or it is black and sticky like tar
-
● unusual weight gain
-
● skin rash or blisters with fever
-
● swelling of the arms and legs, hands and feet
-
● Aspirin is an NSAID medicine but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
-
● Some of these NSAID medicines are sold in lower doses without a prescription (over-the-counter). Talk to your healthcare provider before using over-the-counter NSAIDs for more than 10 days.
**
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Diclofenac SodiumDiclofenac Sodium TABLET, DELAYED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!