Bupropion Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- BUPROPION HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- BUPROPION HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- BUPROPION HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNINGSuicidality and Antidepressant Drugs
Use in Treating Psychiatric Disorders: Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of bupropion or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Bupropion is not approved for use in pediatric patients. (SeeWARNINGS: Clinical Worsening and Suicide Risk in Treating Psychiatric Disorders,PRECAUTIONS: Information for PatientsandPRECAUTIONS: Pediatric Use.)
Use in Smoking Cessation Treatment:
Advise patients and caregivers that the patient using bupropion for smoking cessation should stop taking bupropion and contact a healthcare provider immediately if agitation, hostility, depressed mood, or changes in thinking or behavior that are not typical for the patient are observed, or if the patient develops suicidal ideation or suicidal behavior.
WARNINGS: Neuropsychiatric Symptoms and Suicide Risk in Smoking Cessation TreatmentPRECAUTIONS: Information for Patients
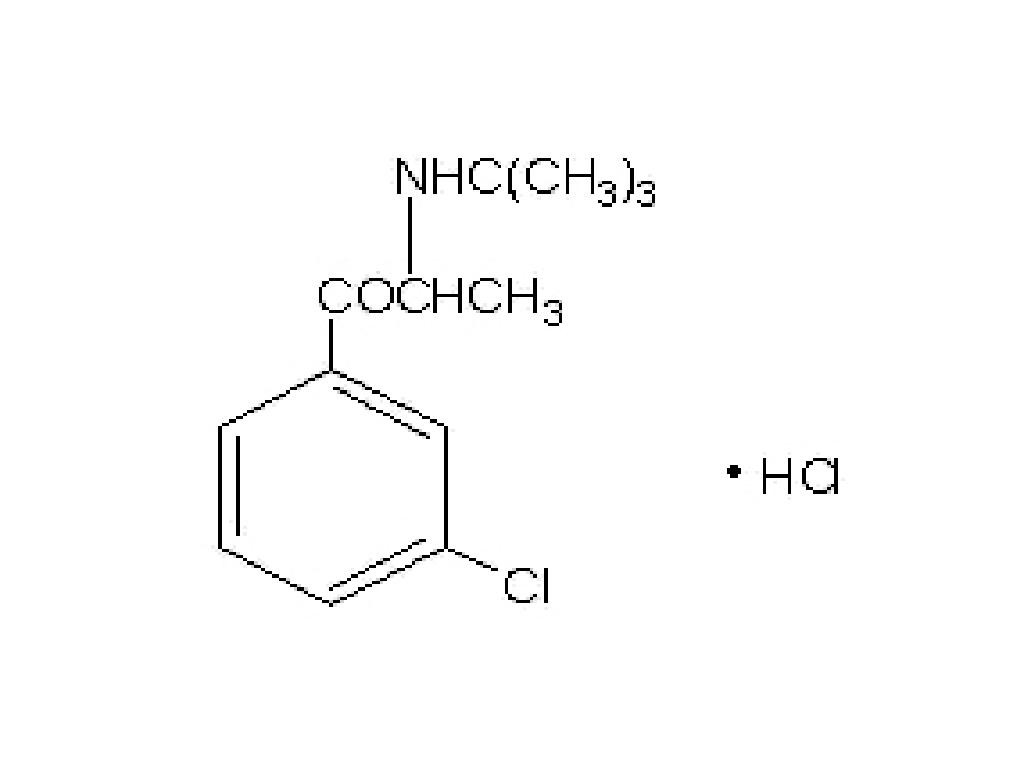
BUPROPION HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
PharmacodynamicsPharmacokinetics
Absorption
Distribution
Metabolism
PRECAUTIONS: Drug Interactions
Elimination
Populations Subgroups
Hepatic
WARNINGSPRECAUTIONSDOSAGE AND ADMINISTRATION
Renal
PRECAUTIONS: General: Renal Impairment
Left Ventricular Dysfunction
Age
PRECAUTIONS: Geriatric Use
Gender
Smokers
INDICATIONS & USAGE
WARNINGSBUPROPION HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Clinical Worsening and Suicide Risk in Treating Psychiatric DisordersAll patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers.
Neuropsychiatric Symptoms and Suicide Risk in Smoking Cessation Treatment
BOXED WARNINGADVERSE REACTIONSThese have included changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, hostility, agitation, aggression, anxiety and panic, as well as suicidal ideation, suicide attempt and completed suicide.
Advise patients and caregivers that the patient using bupropion for smoking cessation should stop taking bupropion and contact a healthcare provider immediately if agitation, depressed mood, or changes in behavior or thinking that are not typical for the patient are observed, or if the patient develops suicidal ideation or suicidal behavior. In many post-marketing cases, resolution of symptoms after discontinuation of ZYBANwas reported, although in some cases the symptoms persisted, therefore, ongoing monitoring and supportive care should be provided until symptoms resolve.
Screening Patients for Bipolar Disorder
Bupropion-Containing Products
Seizures
Bupropion is associated with seizures in approximately 0.4% (4/1,000) of patients treated at doses up to 450 mg/day. This incidence of seizures may exceed that of other marketed antidepressants by as much as 4-fold. This relative risk is only an approximate estimate because no direct comparative studies have been conducted. The estimated seizure incidence for bupropion hydrochloride tablets increases almost 10-fold between 450 and 600 mg/day, which is twice the usually required daily dose (300 mg) and one and one-third the maximum recommended daily dose (450 mg). Given the wide variability among individuals and their capacity to metabolize and eliminate drugs this disproportionate increase in seizure incidence with dose incrementation calls for caution in dosing.
During the initial development, 25 among approximately 2,400 patients treated with bupropion hydrochloride tablets experienced seizures. At the time of seizure, seven patients were receiving daily doses of 450 mg or below for an incidence of 0.33% (3/1,000) within the recommended dose range. Twelve patients experienced seizures at 600 mg/day (2.3% incidence); six additional patients had seizures at daily doses between 600 mg and 900 mg (2.8% incidence).
A separate, prospective study was conducted to determine the incidence of seizure during an 8-week treatment exposure in approximately 3,200 additional patients who received daily doses of up to 450 mg. Patients were permitted to continue treatment beyond 8 weeks if clinically indicated. Eight seizures occurred during the initial 8-week treatment period and five seizures were reported in patients continuing treatment beyond 8 weeks, resulting in a total seizure incidence of 0.4%.
The risk of seizure appears to be strongly associated with dose. Sudden and large increments in dose may contribute to increased risk. While many seizures occurred early in the course of treatment, some seizures did occur after several weeks at fixed dose. Bupropion hydrochloride tablets should be discontinued and not restarted in patients who experience a seizure while on treatment.
The risk of seizure is also related to patient factors, clinical situations, and concomitant medications, which must be considered in selection of patients for therapy with bupropion hydrochloride tablets.
-
● Patient factors: Predisposing factors that may increase the risk of seizure with bupropion use include history of head trauma or prior seizure, central nervous system (CNS) tumor, the presence of severe hepatic cirrhosis, and concomitant medications that lower seizure threshold.
-
● Clinical situations: Circumstances associated with an increased seizure risk include, among others, excessive use of alcohol or sedatives (including benzodiazepines); addiction to opiates, cocaine, or stimulants; use of over-the-counter stimulants and anorectics; and diabetes treated with oral hypoglycemics or insulin.
-
● Concomitant medications: Many medications (e.g., antipsychotics, antidepressants, theophylline, systemic steroids) are known to lower seizure threshold.
Recommendations for Reducing the Risk of Seizure
Retrospective analysis of clinical experience gained during the development of bupropion suggests that the risk of seizure may be minimized if:
-
● the total daily dose of bupropion does not exceed 450 mg,
-
● the daily dose is administered 3 times daily, with each single dose not to exceed 150 mg to avoid high peak concentrations of bupropion and/or its metabolites, and;
-
● the rate of incrementation of dose is very gradual.
Hepatic Impairment
Bupropion should be used with extreme caution in patients with severe hepatic cirrhosis. In these patients a reduced dose and/or frequency is required, as peak bupropion, as well as AUC, levels are substantially increased and accumulation is likely to occur in such patients to a greater extent than usual. The dose should not exceed 75 mg once a day in these patients (seeCLINICAL PHARMACOLOGY,PRECAUTIONS, andDOSAGE AND ADMINISTRATION).
Potential for Hepatotoxicity
PRECAUTIONS
GeneralAgitation and Insomnia
Psychosis, Confusion, and Other Neuropsychiatric Phenomena
Activation of Psychosis and/or Mania
Altered Appetite and Weight
Allergic Reactions
Cardiovascular Effects
Hepatic Impairment
CLINICAL PHARMACOLOGYWARNINGSDOSAGE AND ADMINISTRATION
Renal Impairment
INFORMATION FOR PATIENTS
Clinical Worsening and Suicide Risk in Treating Psychiatric Disorders
Neuropsychiatric Symptoms and Suicide Risk in Smoking Cessation Treatment
Bupropion-Containing Products
LABORATORY TESTS
DRUG INTERACTIONS
Drugs Metabolized by Cytochrome P450IID6 (CYP2D6)
MAO Inhibitors
CONTRAINDICATIONS
Levodopa and Amantadine
Drugs that Lower Seizure Threshold
WARNINGS
Nicotine Transdermal System
PRECAUTIONS: General: Cardiovascular Effects
Alcohol
CONTRAINDICATIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects. Pregnancy Category CLABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
BOX WARNINGWARNINGS: Clinical Worsening and Suicide Risk in Treating Psychiatric DisordersGERIATRIC USE
CLINICAL PHARMACOLOGY
PRECAUTIONS: General: Renal ImpairmentDOSAGE AND ADMINISTRATION
BUPROPION HYDROCHLORIDE ADVERSE REACTIONS
WARNINGSPRECAUTIONSWARNINGSPRECAUTIONS
*
*
Other Events Observed During the Development of Bupropion
WARNINGSPRECAUTIONS
Cardiovascular:
Dermatologic:
Endocrine:
Gastrointestinal:
Genitourinary:
Hematologic/Oncologic:
Musculoskeletal:
Neurological: WARNINGS
Neuropsychiatric:(See PRECAUTIONS
Oral Complaints:
Respiratory:
Special Senses:
Nonspecific:
Postintroduction Reports
Voluntary reports of adverse events temporally associated with bupropion that have been received since market introduction and which may have no causal relationship with the drug include the following:
Body (General):arthralgia, myalgia, and fever with rash and other symptoms suggestive of delayed hypersensitivity. These symptoms may resemble serum sickness (seePRECAUTIONS).
Cardiovascular:hypertension (in some cases severe, seePRECAUTIONS), orthostatic hypotension, third degree heart block
Endocrine:syndrome of inappropriate antidiuretic hormone secretion, hyperglycemia, hypoglycemia
Gastrointestinal:esophagitis, hepatitis, liver damage
Hemic and Lymphatic:ecchymosis, leukocytosis, leukopenia, thrombocytopenia. Altered PT and/or INR, infrequently associated with hemorrhagic or thrombotic complications, were observed when bupropion was coadministered with warfarin.
Musculoskeletal:arthralgia, myalgia, muscle rigidity/fever/rhabdomyolysis, muscle weakness
Nervous:aggression, coma, completed suicide, delirium, dream abnormalities, paranoid ideation, paresthesia, restlessness, suicide attempt, unmasking of tardive dyskinesia
Skin and Appendages:Stevens-Johnson Syndrome, angioedema, exfoliative dermatitis, urticaria
Special Senses:tinnitus, increased intraocular pressure
DRUG ABUSE AND DEPENDENCE
HumansAnimals
OVERDOSAGE
Human Overdose ExperienceOverdosage Management
DOSAGE & ADMINISTRATION
General Dosing ConsiderationsWARNINGS
Usual Dosage for Adults
Increasing the Dosage Above 300 mg/day
Maintenance Treatment
Dosage Adjustment for Patients with Impaired Hepatic Function
CLINICAL PHARMACOLOGYWARNINGSPRECAUTIONS
Dosage Adjustment for Patients with Impaired Renal Function
CLINICAL PHARMACOLOGYPRECAUTIONS
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
BUPROPION HYDROCHLORIDE TABLETS, USPWhat Other Important Information Should I Know About Bupropion Hydrochloride Tablets?
Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Talk to your, or your family member's, healthcare provider about:
-
● all risks and benefits of treatment with antidepressant medicines
-
● all treatment choices for depression or other serious mental illness
1. Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
2. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions.
3. How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
-
● Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
-
● Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
-
● Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
-
● thoughts about suicide or dying
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling very agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
-
● Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
-
● Antidepressants are medicines used to treat depression and other illnesses.It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
-
● Antidepressant medicines have other side effects.Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
-
● Antidepressant medicines can interact with other medicines.Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
-
● Not all antidepressant medicines prescribed for children are FDA approved for use in children.Talk to your child's healthcare provider for more information.
Quitting Smoking, Quit-Smoking Medications, Changes in Thinking and Behavior, Depression, and Suicidal Thoughts or Actions
-
● thoughts about suicide or dying
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● panic attacks
-
● feeling very agitated or restless
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● abnormal thoughts or sensations
-
● seeing or hearing things that are not there (hallucinations)
-
● feeling people are against you (paranoia)
-
● feeling confused
-
● other unusual changes in behavior or mood
What Other Important Information Should I Know About Bupropion Hydrochloride Tablets?
Seizures: There is a chance of having a seizure (convulsion, fit) with bupropion, especially in people:
-
● with certain medical problems.
-
● who take certain medicines.
If you have a seizure while taking bupropion, stop taking the tablets and call your doctor right away.
-
● High blood pressure (hypertension). Some people get high blood pressure, that can be severe, while taking bupropion.The chance of high blood pressure may be higher if you also use nicotine replacement therapy (such as a nicotine patch) to help you stop smoking.
-
● Severe allergic reactions: Some people have severe allergic reactions to bupropion. Stop taking bupropion and call your doctor right awayif you get a rash, itching, hives, fever, swollen lymph glands, painful sores in the mouth or around the eyes, swelling of the lips or tongue, chest pain or have trouble breathing. These could be signs of a serious allergic reaction.
-
● Unusual thoughts or behaviors.Some patients have unusual thoughts or behaviors while taking bupropion, including delusions (believe you are someone else), hallucinations (seeing or hearing things that are not there), paranoia (feeling that people are against you) or feeling confused. If this happens to you, call your doctor.
Who should not take bupropion?
Do not take bupropion if you
-
● have or had a seizure disorder or epilepsy.
-
● are taking ZYBAN(used to help people stop smoking) or any other medicines that contain bupropion hydrochloride, such as WELLBUTRIN SRSustained-Release Tablets or WELLBUTRIN XLExtended-Release Tablets.Bupropion is the same ingredient that is in bupropion hydrochloride tablets, USP.
-
● drink a lot of alcohol and abruptly stop drinking, or use medicines called sedatives (these make you sleepy) or benzodiazepines and you stop using them all of a sudden.
-
● have taken within the last 14 days medicine for depression called a monoamine oxidase inhibitor (MAOI), such as NARDIL(phenelzine sulfate), PARNATE(tranylcypromine sulfate), or MARPLAN(isocarboxazid).
-
● have or had an eating disorder such as anorexia nervosa or bulimia.
-
● are allergic to the active ingredient in bupropion hydrochloride tablets, bupropion, or to any of the inactive ingredients. See the end of this leaflet for a complete list of ingredients in bupropion hydrochloride tablets.
-
● Tell your doctor about your other medical conditions including if you:
-
● are pregnant or plan to become pregnant.It is not known if bupropion can harm your unborn baby.
-
● are breast-feeding.Bupropion passes through your milk. It is not known if bupropion can harm your baby.
-
● have liver problems,especially cirrhosis of the liver.
-
● have kidney problems.
-
● have an eating disorder, such as anorexia nervosa or bulimia.
-
● have had a head injury.
-
● have had a seizure (convulsion, fit).
-
● have a tumor in your nervous system (brain or spine).
-
● have had a heart attack, heart problems, or high blood pressure.
-
● are a diabetic taking insulin or other medicines to control your blood sugar.
-
● drink a lot of alcohol.
-
● abuse prescription medicines or street drugs.
-
● Tell your doctor about all the medicines you take,including prescription and non-prescription medicines, vitamins, and herbal supplements. Many medicines increase your chances of having seizures or other serious side effects if you take them while you are using bupropion.
-
● Take bupropion exactly as prescribed by your doctor.
-
● Take bupropion at the same time each day.
-
● Take your doses of bupropion at least 6 hours apart.
-
● You may take bupropion with or without food.
-
● If you miss a dose, do not take an extra tablet to make up for the dose you forgot. Wait and take your next tablet at the regular time. This is very important. Too much bupropion can increase your chance of having a seizure.
-
● If you take too much bupropion, or overdose, call your local emergency room or poison control center right away.
-
● Do not take any other medicines while using bupropion unless your doctor has told you it is okay.
-
● It may take several weeks for you to feel that bupropion is working. Once you feel better, it is important to keep taking bupropion exactly as directed by your doctor. Call your doctor if you do not feel bupropion is working for you.
-
● Do not change your dose or stop taking bupropion without talking with your doctor first.
-
● Do not drink a lot of alcohol while taking bupropion. If you usually drink a lot of alcohol, talk with your doctor before suddenly stopping. If you suddenly stop drinking alcohol, you may increase your risk of having seizures.
-
● Do not drive a car or use heavy machinery until you know how bupropion affects you. Bupropion can impair your ability to perform these tasks.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store bupropion?
-
● Store bupropion hydrochloride tablets at room temperature. Store out of direct sunlight. Keep bupropion hydrochloride tablets in its tightly closed bottle.
-
● Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use bupropion for a condition for which it was not prescribed. Do not give bupropion to other people, even if they have the same symptoms you have. It may harm them. Keep bupropion out of the reach of children.
What are the ingredients in bupropion hydrochloride tablets?
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Bupropion HydrochlorideBupropion Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!